Pheromone-Binding Proteins in Pest Control: From Molecular Insights to Real-World Applications
- PMID: 40836761
- PMCID: PMC12412164
- DOI: 10.1021/acs.jafc.5c03663
Pheromone-Binding Proteins in Pest Control: From Molecular Insights to Real-World Applications
Abstract
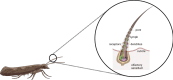
Insects utilize sophisticated olfactory systems to detect chemical cues critical for behaviors such as mating, host selection, and predator avoidance. In lepidopteran moths, sex pheromone communication offers a well-established model in which males detect female-emitted signals over long distances. Central to this process are pheromone-binding proteins (PBPs), which solubilize and transport hydrophobic pheromones through the sensillar lymph to olfactory receptors, enabling precise signal detection. Recent advances in molecular biology, structural biochemistry, and gene-editing technologies such as CRISPR/Cas9 have uncovered nuanced mechanisms underlying PBP function, including ligand-binding specificity, pH-dependent conformational dynamics, and molecular interactions. These discoveries have broad implications, extending beyond chemosensory biology to applications in reverse chemical ecology, biosensing, and environmentally conscious pest control. This review synthesizes insights from in vitro, in silico, and in vivo studies, highlighting the structural and functional diversity of PBPs across species and emphasizing their translational utility as molecular targets for sustainable agriculture and biodiversity conservation.
Keywords: integrated pest management (IPM); lepidopteran sex pheromones; ligand binding; odorant-binding proteins (OBPs); olfaction in insects; olfactory receptors (ORs); pest control strategies; pheromone-binding proteins (PBPs); signal transduction.
Figures





Similar articles
-
Loss of olfaction reduces caterpillar performance and increases susceptibility to a natural enemy.Elife. 2025 Aug 1;14:RP105585. doi: 10.7554/eLife.105585. Elife. 2025. PMID: 40747879 Free PMC article.
-
TabsPBP2, a Pheromone-Binding Protein Highly Expressed in Male Antennae of Tuta absoluta, Binds Sex Pheromones and Tomato Volatiles.Biomolecules. 2025 Aug 11;15(8):1152. doi: 10.3390/biom15081152. Biomolecules. 2025. PMID: 40867597 Free PMC article.
-
Disrupting the olfactory response in Monochamus saltuarius: Two potential target genes for controlling the vector insect of pine wilt disease.Insect Sci. 2025 Jun;32(3):881-898. doi: 10.1111/1744-7917.13431. Epub 2024 Aug 20. Insect Sci. 2025. PMID: 39164734
-
Diversity and role of volatile terpene and terpenoid pheromones in insects.J Econ Entomol. 2025 Feb 11;118(1):9-18. doi: 10.1093/jee/toae271. J Econ Entomol. 2025. PMID: 39578941 Review.
-
Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management.Biology (Basel). 2025 Jul 25;14(8):937. doi: 10.3390/biology14080937. Biology (Basel). 2025. PMID: 40906125 Free PMC article. Review.
References
-
- Greenfield M. D.. Moth Sex-Pheromones - an Evolutionary Perspective. Fla Entomol. 1981;64(1):4–17. doi: 10.2307/3494597. - DOI
-
- Honson N., Gong Y., Plettner E.. Structure and function of insect odorant and pheromone-binding proteins (OBPs and PBPs) and chemosensory-specific proteins (CSPs) Recent Advances in Phytochemistry. 2005;39(05):227–268. doi: 10.1016/S0079-9920(05)80010-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

