A Rapid Assessment Approach for Skin Stratum-Targeted Drug Delivery Systems Using Mass Spectrometry Imaging and Spatial Clustering
- PMID: 40837050
- PMCID: PMC12362830
- DOI: 10.1002/smsc.202500061
A Rapid Assessment Approach for Skin Stratum-Targeted Drug Delivery Systems Using Mass Spectrometry Imaging and Spatial Clustering
Abstract
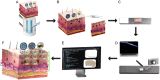
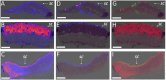
A novel mass spectrometry imaging (MSI)-based concept that enables rapid visualization and evaluation of active pharmaceutical ingredient (API) distribution across skin layers following dermal delivery is presented. This approach integrates desorption electrospray ionization MSI with a newly developed automated computational tool (access provided) that efficiently processes MSI data, isolates skin tissue signals from background interference, and segments the tissue into precise layers. The tool facilitates detailed and rapid assessment of API localization within skin strata in under 10 min per skin specimen. To validate this method, three nanoscale dermal drug delivery systems (DDSs) for the antifungal terbinafine that target distinct skin strata-ethosomes, transethosomes, and microemulsion-are designed and characterized. API permeation in human and porcine skin is evaluated using both manual and automated workflows. The integrated approach demonstrates superior accuracy in skin distribution analysis, a substantial reduction in processing time, and improved efficiency in signal-tissue overlay. Comparative analysis of the DDSs reveals marked differences in drug permeation depth and localization, with transethosomes showing the highest potential for deeper dermal delivery. This method not only provides a powerful tool for DDS evaluation but also enables detailed kinetic studies, offering insights into drug permeation dynamics.
Keywords: dermal delivery; desorption electrospray ionization mass spectrometry imaging; ethosomes; microemulsion; skin layers; terbinafine; transethosomes.
© 2025 The Author(s). Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Unique dermal bacterial signature differentiates atopic dermatitis skin from healthy.mSphere. 2025 Jun 25;10(6):e0015625. doi: 10.1128/msphere.00156-25. Epub 2025 May 9. mSphere. 2025. PMID: 40340458 Free PMC article.
-
Topical antifungal treatments for tinea cruris and tinea corporis.Cochrane Database Syst Rev. 2014 Aug 4;2014(8):CD009992. doi: 10.1002/14651858.CD009992.pub2. Cochrane Database Syst Rev. 2014. PMID: 25090020 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Parsi K., Partsch H., Rabe E., Ramelet A. A., Australas. J. Dermatol. 2011, 52, 159. - PubMed
-
- Godin B., Touitou E., Rubinstein E., Athamna A., Athamna M., J. Antimicrob. Chemother. 2005, 55, 989. - PubMed
-
- Walsh T. J., Dixon D. M., Med. Microbiol. 1996, 75, 1.
-
- In Vitro Permeation Test Studies for Topical Drug Products Submitted in ANDAs Guidance for Industry, U.S. department of Health and Human Services Food and Drug Administration and Center for Drug Evaluation and Research (CDER), 2022, Center for Drug Evaluation and Research, Office of Regulatory Policy.
LinkOut - more resources
Full Text Sources
