Efficient Electrochemical CO2 Reduction Using AgN3 Single-Atom Sites Embedded in Free-Standing Electrodes for Flow Cell Applications
- PMID: 40837051
- PMCID: PMC12362748
- DOI: 10.1002/smsc.202400643
Efficient Electrochemical CO2 Reduction Using AgN3 Single-Atom Sites Embedded in Free-Standing Electrodes for Flow Cell Applications
Abstract
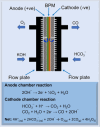
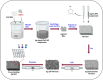
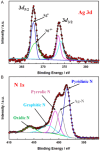
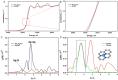
The electrochemical reduction of CO2 into valuable chemicals presents a promising strategy for carbon utilization; however, it remains challenging due to low activity, poor selectivity and stability of existing catalysts. In this study, we report the fabrication of free-standing silver single-atom catalysts (Ag SACs) designed for the efficient conversion of CO2 to carbon monoxide (CO) at high current densities in a bicarbonate electrolyzer. The Ag single atoms dispersed within a carbon matrix, forming Ag-N3 active sites for the electrocatalytic CO2 reduction reaction (CO2 RR). The catalytic activity and stability of the free-standing Ag SACs are evaluated at a current density of 100 mA cm-2, demonstrating prolonged electrolysis with consistent Faradaic efficiency for CO production. Density functional theory calculations revealed that the Ag-N3 active site significantly lowers the energy barriers for the CO2 absorption step compared to Ag-Ag and Ag-Ni sites, facilitating CO2 activation and contributing to enhanced catalytic activity and stability during CO2 reduction. Detailed analysis of the electronic structure and coordination environment further validates the superior performance of the Ag-N3 site in the free-standing Ag SACs, underscoring their effectiveness in CO2 electroreduction. These findings highlight the potential of the free-standing Ag SACs to advance CO2 reduction technologies, offering enhanced efficiency and selectivity for CO2 conversion.
Keywords: CO2 reduction; electrolysis; free‐standing electrodes; silver; single‐atom catalysts.
© 2025 The Author(s). Small Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lin S., Diercks C. S., Zhang Y.‐B., Kornienko N., Nichols E. M., Zhao Y., Paris A. R., Kim D., Yang P., Yaghi O. M., Sci. 1979 2015, 349, 1208. - PubMed
-
- Sun Z., Talreja N., Tao H., Texter J., Muhler M., Strunk J., Chen J., Angew. Chem., Int. Ed. 2018, 57, 7610. - PubMed
-
- Ross M. B., De Luna P., Li Y., Dinh C. T., Kim D., Yang P., Sargent E. H., Nat Catal 2019, 2, 648.
-
- De Luna P., Hahn C., Higgins D., Jaffer S. A., Jaramillo T. F., Sargent E. H., Sci. 1979 2019, 364, eaav3506. - PubMed
-
- Zhang Y.‐J., Sethuraman V., Michalsky R., Peterson A. A., ACS Catal 2014, 4, 3742.
LinkOut - more resources
Full Text Sources
