ADAMTS2: More than a procollagen N-proteinase
- PMID: 40837410
- PMCID: PMC12362006
- DOI: 10.1016/j.gendis.2025.101686
ADAMTS2: More than a procollagen N-proteinase
Abstract
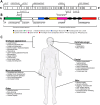
A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAMTS2) is a member of the ADAMTS zinc metalloproteinase family, best known for its role as a procollagen I N-proteinase in the maturation of fibrillar collagens. Biallelic defects in the ADAMTS2 gene, resulting in a loss of ADAMTS2 enzyme activity and consequent retention of N-propeptides in type I procollagen molecules, lead to the rare monogenic disease Ehlers-Danlos syndrome dermatosparaxis type (dEDS) in humans, and dermatosparaxis in animals, conditions that are hallmarked by extreme fragility of the skin and other soft connective tissues. Recent studies have expanded the substrate repertoire of ADAMTS2 considerably, revealing its potential implication in several biological processes, including angiogenesis, lymphangiogenesis, neurodevelopment, immunity, and spermatogenesis. There is also emerging evidence for a role for ADAMTS2 in complex disorders, including cancer and cardiovascular and neurodegenerative disease. These findings may not only provide answers to hitherto unsolved questions in dermatosparaxis but also unveil a therapeutic and/or biomarker potential of ADAMTS2 in many diseases. This narrative review provides an in-depth overview of the discovery, structure, regulation, and enzymatic role of ADAMTS2, its role in fibrillar collagen maturation and in dEDS pathogenesis, as well as its newly discovered substrates and its potential role in complex disorders.
Keywords: ADAMTS2; Collagen; Dermatosparaxis; Ehlers-Danlos syndrome; Procollagen N-Proteinase.
© 2025 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltdé.
Conflict of interest statement
Anne-Marie Malfait is a consultant for Orion, Averitas, Eli Lilly, and Novartis.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The ADAMTS2 metalloproteinase inhibits tumor growth by regulating the innate immune system.Cancer Cell Int. 2025 Jun 23;25(1):229. doi: 10.1186/s12935-025-03880-1. Cancer Cell Int. 2025. PMID: 40551102 Free PMC article.
-
Dermal pathology in a Catahoula Leopard dog with Dermasparaxis Ehlers Danlos syndrome caused by a homozygous ADAMTS2 missense variant.Top Companion Anim Med. 2025 May-Jun;66:100976. doi: 10.1016/j.tcam.2025.100976. Epub 2025 Mar 12. Top Companion Anim Med. 2025. PMID: 40086505
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
AGA Clinical Practice Update on GI Manifestations and Autonomic or Immune Dysfunction in Hypermobile Ehlers-Danlos Syndrome: Expert Review.Clin Gastroenterol Hepatol. 2025 Jul;23(8):1291-1302. doi: 10.1016/j.cgh.2025.02.015. Epub 2025 May 19. Clin Gastroenterol Hepatol. 2025. PMID: 40387691 Review.
References
-
- Ruggiero F. Springer International Publishing; Cham: 2021. The Collagen Superfamily and Collagenopathies.
-
- Kadler K.E., Baldock C., Bella J., Boot-Handford R.P. Collagens at a glance. J Cell Sci. 2007;120(pt 12):1955–1958. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

