Activity in an air-liquid interface lung infection model, feasibility of inhaled delivery, and stability of cell-free supernatants from Lacticaseibacillus rhamnosus against Pseudomonas aeruginosa pulmonary infections
- PMID: 40838010
- PMCID: PMC12361147
- DOI: 10.3389/fmicb.2025.1630017
Activity in an air-liquid interface lung infection model, feasibility of inhaled delivery, and stability of cell-free supernatants from Lacticaseibacillus rhamnosus against Pseudomonas aeruginosa pulmonary infections
Abstract
Objective: Given the increasing prevalence of multidrug-resistant pathogens and the diminishing efficacy of conventional antibiotics, this study explores the potential of probiotics or their metabolic products as alternative antimicrobial agents. Specifically, we investigated the antibacterial properties of cell-free supernatants (CFS) derived from the probiotic strain Lacticaseibacillus rhamnosus GG for the local treatment of Pseudomonas aeruginosa lung infections.
Methods: To simulate the human respiratory environment, we employed various in vitro models. The cytotoxicity and antibacterial activity of CFS were assessed using an Air-Liquid Interface (ALI) lung infection model based on differentiated NCI-H441 human distal lung epithelial cells cultured on Transwell® inserts. To evaluate the feasibility of aerosol-based delivery, we developed and characterized a liquid formulation of CFS. The aerodynamic performance of nebulized CFS was analyzed using a twin-stage impinger (TSI) and a Next Generation Impactor (NGI), the latter equipped with a breathing simulator to mimic respiratory profiles of both healthy individuals and cystic fibrosis patients. Additionally, the physicochemical and biological stability of CFS was assessed under various storage conditions.
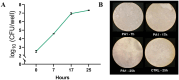
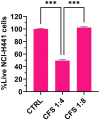
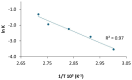
Results: CFS demonstrated significant antibacterial activity in the ALI model, reducing P. aeruginosa colony-forming units by up to 3 log units after 7 h of incubation, without inducing cytotoxic effects. Scanning electron microscopy confirmed these findings. Aerodynamic testing with the TSI and an Aerogen® mesh nebulizer showed that 76% of the nebulized product was deposited in the second stage, indicating effective deep lung delivery. NGI analysis revealed a favorable aerodynamic particle size distribution (APSD), with a fine particle fraction (FPF) exceeding 60% and a mass median aerodynamic diameter (MMAD) suitable for deep airway deposition. Physicochemical stability studies under stressed temperature conditions predicted prolonged physical stability for CFS at 25°C and demonstrated that they retained anti-pseudomonal activity after 1 year of storage at room temperature, 4°C, and -20°C.
Conclusion: These findings support the potential of L. rhamnosus GG-derived CFS as a promising candidate for inhaled therapy against P. aeruginosa lung infections. Further validation in animal models is warranted to confirm its therapeutic efficacy and safety in vivo, potentially contributing to the development of novel localized treatment strategies for respiratory infections.
Keywords: Lacticaseibacillus rhamnosus; Pseudomonas aeruginosa; cystic fibrosis; lung infections; lung model; solution for inhalation.
Copyright © 2025 Piras, Bianchi, Bona, Grassiri, Kaya, Bertacca, Migone, Maisetta, Esin and Batoni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
-
- Aggarwal S., Sabharwal V., Kaushik P., Joshi A., Aayushi A., Suri M. (2022). Postbiotics: from emerging concept to application. Front. Sustain. Food Syst. 6:887642. doi: 10.3389/fsufs.2022.887642 - DOI
LinkOut - more resources
Full Text Sources

