Photocyclization of Alkenes and Arenes: Penetrating Through Aromatic Armor with the Help of Excited State Antiaromaticity
- PMID: 40838095
- PMCID: PMC12363374
- DOI: 10.3390/chemistry7030079
Photocyclization of Alkenes and Arenes: Penetrating Through Aromatic Armor with the Help of Excited State Antiaromaticity
Abstract
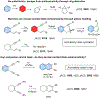
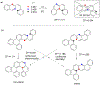
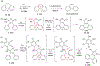
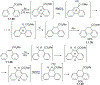
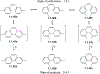
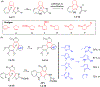
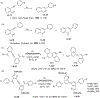
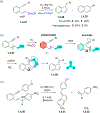
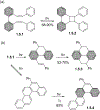
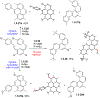
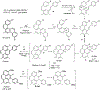
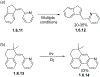
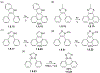
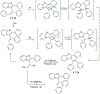
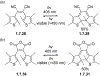
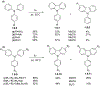
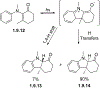
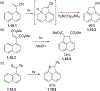
This review focuses on photocyclization reactions involving alkenes and arenes. Photochemistry opens up synthetic opportunities difficult for thermal methods, using light as a versatile tool to convert stable ground-state molecules into their reactive excited counterparts. This difference can be particularly striking for aromatic molecules, which, according to Baird's rule, transform from highly stable entities into their antiaromatic "evil twins". We highlight classical reactions, such as the photocyclization of stilbenes, to show how alkenes and aromatic rings can undergo intramolecular cyclizations to form complex structures. When possible, we explain how antiaromaticity develops in excited states and how this can expand synthetic possibilities. The review also examines how factors such as oxidants, substituents, and reaction conditions influence product selectivity, providing useful insights for improving reaction outcomes and demonstrating how photochemical methods can drive the development of new synthetic strategies.
Keywords: antiaromaticity; arenes; photocyclization.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflicts of interest.
Figures
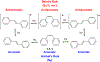
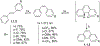

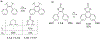
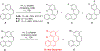
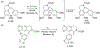
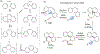

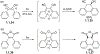
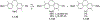
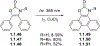
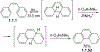

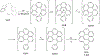

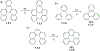
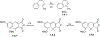
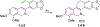
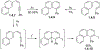
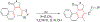
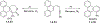
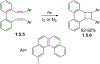
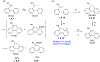
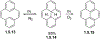
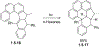


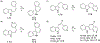
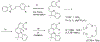
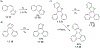


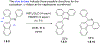


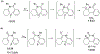

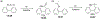
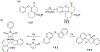
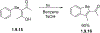
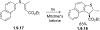
References
-
- Hu C; Mena J; Alabugin IV Design Principles of the Use of Alkynes in Radical Cascades. Nat. Rev. Chem 2023, 7, 405–423. - PubMed
-
- Nguyen MT; Matus MH; Lester William A; Dixon DA Heats of Formation of Triplet Ethylene, Ethylidene, and Acetylene. J. Phys. Chem. A 2008, 112, 2082–2087. - PubMed
-
- Zimmerman HE; Alabugin IV Excited State Energy Distribution and Redistribution and Chemical Reactivity; Mechanistic and Exploratory Organic Photochemistry1,2. J. Am. Chem. Soc 2000, 122, 952–953.
-
- Modern Molecular Photochemistry of Organic Molecules. University Science Books. Available online: https://uscibooks.aip.org/books/modern-molecular-photochemistry-of-organ... (accessed on 24 January 2025).
Grants and funding
LinkOut - more resources
Full Text Sources
