Three-Dimensional Magnetic Bioprinting Spheroids as an In Vitro Model to Study the Oviductal Physiology
- PMID: 40838423
- PMCID: PMC12368830
- DOI: 10.1002/mrd.70049
Three-Dimensional Magnetic Bioprinting Spheroids as an In Vitro Model to Study the Oviductal Physiology
Abstract
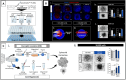
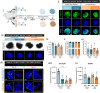
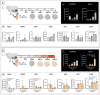
In vitro models to study the oviduct are challenged by cellular dedifferentiation, a complex coculture system for embryo production, limited cell lifespan, and/or very complex methodologies. Hence, we aimed to develop an in vitro oviductal model using the magnetic bioprinting system, a three-dimensional (3D) culture system. Using the bovine epithelial and stromal oviductal cells (BOEC and BOSC, respectively), we produced the Oviductal Magnetic Spheroid (OMS), a duo somatic cell spheroid aggregate with self-organization capacity. The OMS showed to be viable for 21 days and recapitulated the oviductal tissue features after 7 days in culture, such as a simple epithelial cell layer facing outwards, expressing ciliation (acetylated tubulin positive) and secretory marker (oviduct-specific glycoprotein 1). Although the responsiveness for hormonal treatment with estradiol and progesterone in an estrous cycle-dependent way might require further improvements, the OMS offers an ethical and practical alternative as a three-dimensional oviductal in vitro model to study oviductal physiology, and maybe, a future platform to test therapies and a technology aiming to improve fertility and assisted reproduction success.
Keywords: OVGP1; bioprinting; cytokeratin; extracellular matrix; vimentin.
© 2025 The Author(s). Molecular Reproduction and Development published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Generation of Oviductal Glycoprotein 1 Cre Mouse Model for the Study of Secretory Epithelial Cells of the Oviduct.Endocrinology. 2024 May 27;165(7):bqae070. doi: 10.1210/endocr/bqae070. Endocrinology. 2024. PMID: 38916490 Free PMC article.
-
Gelatin vs GelMA in alginate-based bioinks as a platform for versatile 3D bioprintable in vitro systems.Biomater Adv. 2025 Dec;177:214408. doi: 10.1016/j.bioadv.2025.214408. Epub 2025 Jul 9. Biomater Adv. 2025. PMID: 40669326
-
Role of steroid hormones in the maintenance of focal adhesions in bovine oviductal epithelial cells.Domest Anim Endocrinol. 2024 Jul;88:106839. doi: 10.1016/j.domaniend.2024.106839. Epub 2024 Feb 29. Domest Anim Endocrinol. 2024. PMID: 38433026
-
Magnetic-Based Human Tissue 3D Cell Culture: A Systematic Review.Int J Mol Sci. 2022 Oct 21;23(20):12681. doi: 10.3390/ijms232012681. Int J Mol Sci. 2022. PMID: 36293537 Free PMC article.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
References
-
- Bauersachs, S. , Rehfeld S., Ulbrich S., et al. 2004. “Monitoring Gene Expression Changes in Bovine Oviduct Epithelial Cells During the Oestrous Cycle.” Journal of Molecular Endocrinology 32, no. 2: 449–466. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

