Connexin 43 Role in Mitochondrial Transfer and Homeostasis in the Central Nervous System
- PMID: 40838510
- PMCID: PMC12368938
- DOI: 10.1002/jcp.70086
Connexin 43 Role in Mitochondrial Transfer and Homeostasis in the Central Nervous System
Abstract
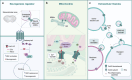
Connexin 43 (Cx43) is a transmembrane protein involved in the assembly of gap junctions (GJs) and hemichannels (HCs), organized structures that allow the transferring of ions and small signaling molecules between cells and/or extracellular environment, thereby contributing to tissue homeostasis intercellular communication. Cx43 has recently been identified within the mitochondria of cells, suggesting that it may have additional functions beyond its canonical role. Most studies of mitochondrial Cx43 (mt-Cx43) have been limited to cells of the cardiovascular system, where it appears to play a role in ATP production, calcium homeostasis, and the response to oxidative stress. However, its functions within the central nervous system (CNS) are not fully understood. Recently, it has been observed that Cx43-forming GJs is one of the key mechanisms that cells use for the transfer of organelles, including mitochondria. Cx43-mediated mitochondrial transfer is crucial in the CNS, supporting cellular homeostasis and neuroprotection under both physiological and pathological conditions. The dual roles of Cx43 in regulating mitochondrial function and in mediating mitochondrial transfer, raise important questions about how it coordinates these mechanisms. Herein, we reviewed recent findings on the importance of Cx43 and mt-Cx43 in the healthy and altered CNS environment, with the aim of shedding light on its potential role in CNS homeostasis and as a therapeutic target in neurological disorder in which Cx43 plays a predominant function.
Keywords: gap junction; homeostasis; intercellular communication; metabolism; neurological disorder.
© 2025 The Author(s). Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Gap junction connexins in female reproductive organs: implications for women's reproductive health.Hum Reprod Update. 2015 May-Jun;21(3):340-52. doi: 10.1093/humupd/dmv007. Epub 2015 Feb 9. Hum Reprod Update. 2015. PMID: 25667189
-
Connexin-43 remodelling and arrhythmias: hemichannels as key drivers of cardiac dysfunction.J Physiol. 2025 Aug;603(15):4293-4306. doi: 10.1113/JP288091. Epub 2025 May 5. J Physiol. 2025. PMID: 40320914 Review.
-
Connexin 43 regulates intercellular mitochondrial transfer from human mesenchymal stromal cells to chondrocytes.Stem Cell Res Ther. 2024 Oct 10;15(1):359. doi: 10.1186/s13287-024-03932-9. Stem Cell Res Ther. 2024. PMID: 39390589 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

