Detection of Neuronal Glutamate in Brain Extracellular Space In Vivo Using Microdialysis and Metabolic Labeling with Glutamine
- PMID: 40838767
- PMCID: PMC12418293
- DOI: 10.1021/acschemneuro.5c00518
Detection of Neuronal Glutamate in Brain Extracellular Space In Vivo Using Microdialysis and Metabolic Labeling with Glutamine
Abstract
Extracellular glutamate (Glu) concentration measured in the brain using microdialysis sampling is regulated differently from that expected for classical neurotransmitters; e.g., the basal Glu concentration is not affected by blocking action potentials. Additionally, other sources, such as glial cells, contribute to Glu extracellular concentration making it difficult to interpret detected changes. We have found that infusing 2.5 μM 13C5-glutamine (Gln) through a microdialysis probe inserted in the rat cortex results in collection of 144 ± 35 nM (n = 11) 13C5-Glu in dialysate. The recovered 13C5-Glu was reduced by 33% by infusion of 20 mM α-(methylamino)isobutyric acid and 58% by 500 mM riluzole, inhibitors of glutamine transport into neurons. The 13C5-Glu measured was reduced by 62% with tetrodotoxin (TTX), a sodium channel blocker, and 59% with (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), a metabotropic glutamate agonist, while endogenous Glu remained unchanged. These results support the hypothesis that the measured 13C5-Glu is derived from neurons via the Gln-Glu shuttle. To further investigate regulation of 13C5-Glu, we applied a stressor (tail pinch), observing a 155% increase in dialysate 13C5-Glu concentration. This effect was blocked by infusion of TTX indicating neuronal release. Local infusion of l-trans-pyrrolidine-2,4-dicarboxylic acid (PDC), a Glu uptake inhibitor, increased both endogenous Glu and 13C5-Glu concentrations, consistent with reverse transport and spread of neuronal release. Taken together, these experiments show that metabolic labeling of Glu via Gln delivered through a microdialysis probe allows differentiation of neuronal and other sources of Glu in the brain. The results support the concept of compartmentalized Glu in the brain.
Keywords: astrocyte; glutamate-glutamine shuttle; mass spectrometry; microdialysis; neuronal glutamate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




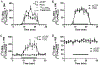


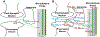
References
-
- Araque A; Parpura V; Sanzgiri RP; Haydon PG Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences 1999, 22 (5), 208–215. - PubMed
-
- Del Arco A; Segovia G; Fuxe K; Mora F Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? Journal of Neurochemistry 2003, 85 (1), 23–33. - PubMed
-
- Haydon PG Glia: Listening and talking to the synapse. Nat. Rev. Neurosci 2001, 2 (3), 185–193. - PubMed
-
- Clements JD; Lester RAJ; Tong G; Jahr CE; Westbrook GL The time course of glutamate in the synaptic cleft. Science 1992, 258 (5087), 1498–1501. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

