The Acute Effects of Cannabidiol on Physiological and Subjective Responses to Endurance Exercise: A Dose-Ranging Randomised Controlled Crossover Trial
- PMID: 40839290
- PMCID: PMC12370602
- DOI: 10.1186/s40798-025-00895-w
The Acute Effects of Cannabidiol on Physiological and Subjective Responses to Endurance Exercise: A Dose-Ranging Randomised Controlled Crossover Trial
Abstract
Background: Athletes report using cannabidiol (CBD), a non-intoxicating constituent of Cannabis sativa L., to enhance post-exercise recovery and manage other health conditions (e.g., poor sleep, anxiety, concussion). However, whether CBD influences performance-related outcomes remains unclear. This study investigated the acute effects of a low, nutraceutical (50 mg) and moderate, therapeutic (300 mg) dose of CBD on physiological and subjective responses to endurance exercise in trained runners.
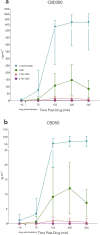
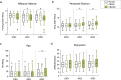
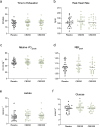
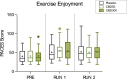
Results: A randomised double-blind, placebo-controlled, crossover clinical trial was conducted at the University of Sydney between 24th October 2022 and 4th March 2024. Twenty-five participants (16 male; O2max = 53.1 ± 7.5 mL·min kg-1) received either 0 (placebo), 50 or 300 mg CBD 1.5 h prior to completing a 60-min, submaximal intensity (~ 70% O2max) treadmill run (RUN 1), followed by an incremental run to volitional exhaustion (RUN 2). Neither dose of CBD altered subjective responses (i.e., affective valence, enjoyment, perceived exertion, pain) during RUN 1, nor enjoyment, mood or anxiety following RUN 1 and 2 (p's > 0.05). CBD also had a limited effect on exercise physiology and performance, with heart rate, exercise efficiency (i.e., O2), O2peak and time to exhaustion (TTE) unchanged relative to placebo (p's > 0.05). However, 300 mg CBD decreased the respiratory exchange ratio during RUN 1 (p = 0.030) and 50 mg CBD increased blood glucose upon cessation of RUN 2 (p = 0.003), compared to placebo. There was no effect of either dose on plasma concentrations of muscle damage markers, creatine and myoglobin (p's > 0.05), but a Treatment x Time x Sex interaction was identified for the gastrointestinal barrier function marker, lipopolysaccharide, with post hoc analyses revealing higher concentrations in females Post RUN 2 on 50 mg (p = 0.032), but not 300mg CBD (p = 1.000), compared to placebo.
Conclusions: CBD (50 mg, 300 mg; acute) does not appear to alter the subjective experience of submaximal intensity exercise, impact endurance performance (i.e., TTE) or have compelling effects on physiological responses to exercise. Use of CBD by athletes is, therefore, unlikely to be ergolytic or ergogenic at low to moderate doses.
Trial registration: The trial was approved by the Sydney Local Health District's Human Research Ethics Committee (2021/ ETH11945; X21-0392) and registered prospectively with the Australia and New Zealand Clinical Trials Registry (ACTRN12622000717752).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval and Consent to Participate: This study was approved by the Sydney Local Health District’s Human Research Ethics Committee (2021/ ETH11945; X21-0392), registered prospectively with the Australia and New Zealand Clinical Trials Registry (ACTRN12622000717752) on 19th May, 2022 and conducted in accordance with the standards of ethics outlined in the Declaration of Helsinki. All participants provided written informed consent prior to enrolment. Consent for Publication: Not applicable. Competing interests: A.S., R.G., K.W., I.S.M., and D.M. receive salary support from the Lambert Initiative for Cannabinoid Therapeutics. I.S.M. and D.M. has received consulting fees from Medicinal Cannabis Industry Australia (MCIA). I.S.M. has also received consulting fees Kinoxis Therapeutics and Janssen. I.S.M. and D.M. have served as expert witnesses in medicolegal cases involving cannabis and cannabinoids. I.S.M is also named as an inventor on several patents relating to novel cannabinoid therapeutics (PCT/AU2018/05089 and PCT/AU2019/050554).
Figures








References
-
- Hanus LO, et al. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357–92. - PubMed
-
- McCartney D, et al. The effect of cannabidiol on simulated car driving performance: a randomised, double-blind, placebo-controlled, crossover, dose-ranging clinical trial protocol. Hum Psychopharmacol Clin Exp. 2020;35(5): e2749. - PubMed
LinkOut - more resources
Full Text Sources

