Mesenchymal Stem Cells Expressing CES1 and Soluble TRAIL Activate CPT-11 and Induce Apoptosis in Lung Cancer Brain Metastatic Lesions
- PMID: 40839370
- PMCID: PMC12417980
- DOI: 10.1158/2767-9764.CRC-25-0209
Mesenchymal Stem Cells Expressing CES1 and Soluble TRAIL Activate CPT-11 and Induce Apoptosis in Lung Cancer Brain Metastatic Lesions
Abstract
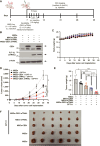
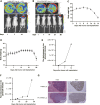
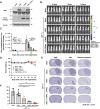
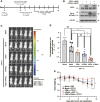
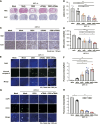
We aimed to develop a novel therapeutic strategy for lung cancer brain metastases by leveraging the tumor-tropic properties of genetically engineered Wharton's Jelly-derived mesenchymal stem cells (WJ-MSC) as vehicles for dual-agent gene therapy across the blood-brain barrier. WJ-MSCs were transiently engineered using lipid nanoparticle technology to coexpress soluble TRAIL (sTRAIL) and the prodrug-activating enzyme carboxylesterase 1 (CES1). In vitro analyses assessed transfection efficiency, therapeutic protein expression, apoptosis induction, and maintenance of stemness. Tumor-homing capacity was evaluated via EGFP labeling and intracerebral tracking. Therapeutic efficacy was tested in subcutaneous and intracerebral lung cancer xenograft models using bioluminescent imaging, histopathology, and IHC. In vivo treatment included intraperitoneal CPT-11 administration to assess synergy between CES1-mediated prodrug activation and sTRAIL-induced apoptosis. Modified WJ-MSCs exhibited preserved stem cell characteristics and strong tropism toward brain tumor sites. They secreted high levels of functional sTRAIL and CES1, enabling local activation of CPT-11 into SN-38 and inducing apoptosis through death receptor signaling (DR4/DR5). Combination therapy with WJ-MSCs-CES1.sTRAIL and CPT-11 significantly suppressed tumor growth in lung cancer brain metastasis models compared with control groups. The approach demonstrated selective cytotoxicity, minimal off-target effects, and favorable safety profiles. This study establishes a nonviral, transient gene delivery platform using autologous WJ-MSCs for dual-action gene therapy in lung cancer brain metastases. The combined use of CES1 and sTRAIL and enables precise tumor targeting and drug activation, offering a promising avenue for personalized, stem cell-based treatment strategies to improve outcomes in patients with brain metastatic lung cancer.
Significance: This study presents a nonviral, stem cell-based therapy for brain metastatic non-small cell lung cancer using WJ-MSCs expressing sTRAIL and CES1. These engineered cells home to tumors, activate CPT-11, and induce apoptosis. The dual-action strategy significantly reduced brain tumor burden with minimal toxicity, demonstrating strong therapeutic potential.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
No disclosures were reported.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

