Essential role of protein kinase R in the pathogenesis of pulmonary veno-occlusive disease
- PMID: 40839386
- PMCID: PMC12513476
- DOI: 10.1172/jci.insight.193495
Essential role of protein kinase R in the pathogenesis of pulmonary veno-occlusive disease
Abstract
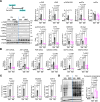
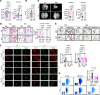
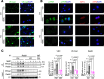
Pulmonary veno-occlusive disease (PVOD) is a rare and severe subtype of pulmonary arterial hypertension, characterized by progressive remodeling of small pulmonary arteries and veins with no therapies. Using a mitomycin C-induced (MMC-induced) rat model, we previously demonstrated that protein kinase R-mediated (PKR-mediated) integrated stress response (ISR) drives endothelial dysfunction and vascular remodeling. To determine whether PKR is the primary mediator of ISR and the pathogenesis, we treated control (Ctrl) and PKR-knockout (KO) mice with the same dose of MMC. Consistent with rat data, Ctrl mice displayed ISR activation, vascular remodeling, and pulmonary hypertension after MMC treatment, while KO mice showed none of these phenotypes. Proteomic analysis revealed that MMC-mediated ISR activation attenuated protein synthesis in Ctrl but not in KO mice. These findings underscore the critical role of PKR-dependent ISR activation and subsequent perturbation of proteostasis as central mechanisms driving PVOD pathogenesis and identify PKR as a promising therapeutic target.
Keywords: Cell biology; Cell stress; Vascular biology.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

