GABAergic interneurons contribute to the fatal seizure phenotype of CLN2 disease mice
- PMID: 40839402
- PMCID: PMC12513480
- DOI: 10.1172/jci.insight.184487
GABAergic interneurons contribute to the fatal seizure phenotype of CLN2 disease mice
Abstract
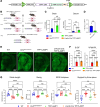
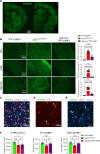
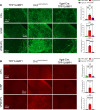
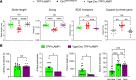
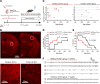
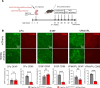
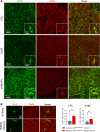
The cellular etiology of seizures in CLN2 disease, a childhood-onset neurodegenerative lysosomal storage disorder caused by a deficiency of tripeptidyl peptidase 1 (TPP1), remains elusive. Given that Cln2R207X/R207X mice display fatal spontaneous seizures and an early loss of several cortical GABAergic interneuron populations, we hypothesized that these 2 events might be causally related. To study the cell-autonomous effects of interneuron-specific TPP1 deficiency, we first generated transgenic mice expressing loxP-flanked lysosomal membrane-tethered TPP1 (TPP1LAMP1 mice) on the Cln2R207X/R207X genetic background, and then crossed TPP1LAMP1 mice with Vgat-Cre mice. These Vgat-Cre; TPP1LAMP1 mice accumulated storage material in cortical and striatal interneurons. Vgat-Cre; TPP1LAMP1 mice also died more readily after pentylenetetrazole-induced seizures, indicating that interneuron-specific TPP1 deficiency renders these mice more susceptible to seizure-induced mortality. We also selectively activated interneurons using designer receptors exclusively activated by designer drugs (DREADDs) in Vgat-Cre; Cln2R207X/R207X mice. Electroencephalogram monitoring revealed that DREADD-mediated activation of interneurons markedly accelerated the onset of spontaneous seizures and seizure-associated death in Vgat-Cre; Cln2R207X/R207X mice, suggesting that modulating interneuron activity can exacerbate epileptiform abnormalities. Taken together, these results provide mechanistic insights into the underlying etiology of seizures and premature death that characterize CLN2 disease.
Keywords: Genetic diseases; Genetics; Lysosomes; Neuroscience; Seizures.
Conflict of interest statement
Figures
Update of
-
GABAergic interneurons contribute to the fatal seizure phenotype of CLN2 disease mice.bioRxiv [Preprint]. 2024 Mar 30:2024.03.29.587276. doi: 10.1101/2024.03.29.587276. bioRxiv. 2024. Update in: JCI Insight. 2025 Aug 21;10(19):e184487. doi: 10.1172/jci.insight.184487. PMID: 38585903 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

