Gilteritinib in FLT3-mutated acute myeloid leukemia: A real-world Italian experience
- PMID: 40839405
- PMCID: PMC12369923
- DOI: 10.1002/cncr.70055
Gilteritinib in FLT3-mutated acute myeloid leukemia: A real-world Italian experience
Abstract
Background and methods: This real-world study evaluated the clinical effectiveness of gilteritinib in 205 patients with relapsed or refractory (R/R) FLT3-mutated acute myeloid leukemia (AML) enrolled in the Italian expanded access since January 2018.
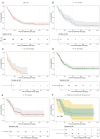
Results: Of the 205 patients, 124 (60.5%) received gilteritinib as a bridging therapy to allogeneic stem cell transplantation (allo-SCT), achieving complete remission in 52.4% (n = 65). The median overall survival (OS) for the entire cohort was 11.0 months, with estimated OS rates of 46.8% at 1 year and 28.5% at 3 years. Sixty patients (48% of those bridged) underwent allo-SCT after a median of 3.7 months on gilteritinib, achieving posttransplant OS rates of 65.2% at 1 year and 56.1% at 3 years. The acquisition of FLT3 mutations at relapse and the presence of TP53 co-mutations were significantly associated with inferior outcomes. Among 46 patients (22.4%) who relapsed after allo-SCT, gilteritinib treatment yielded an overall response rate (ORR) of 54.3%, a median OS of 11.1 months, and 1- and 3-year OS rates of 49.5% and 15.5%, respectively. Additionally, 35 patients (17.1%) previously treated with nonintensive chemotherapy received gilteritinib until disease progression or intolerance, achieving an ORR of 11.4%, a median OS of 5.9 months, and a 1-year OS rate of 29.0%.
Conclusions: These real-world data confirm that clinical outcomes achieved with gilteritinib in patients with R/R FLT3-mutated AML are consistent with those observed in pivotal clinical trials. Notably, approximately half of the transplant-eligible patients were successfully bridged to allo-SCT and demonstrated encouraging long-term survival.
Keywords: FLT3; acute myeloid leukemia; gilteritinib; real‐world; tyrosine kinase inhibitor.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Roberto Cairoli reports consulting fees from AbbVie, Janssen Global Services, LLC, and Novartis; and fees for professional activities from Astellas Pharma, Jazz Pharmaceuticals, and Kite Pharma, Inc. Anna Candoni reports consulting fees from Amgen, Jazz Pharmaceuticals, Pfizer, and Servier Pharmaceuticals LLC; and fees for professional activities from AbbVie, Astellas Pharma, and Incyte. Nicola Fracchiolla reports fees for professional activities from AbbVie. Sara Galimberti reports grant and/or contract funding from AbbVie; and fees for travel from Jazz Pharmaceuticals and Novartis. Pellegrino Musto reports fees for professional activities from Novartis. Cristina Papayannidis reports fees for professional activities from AbbVie, Amgen, Astellas Pharma, Blueprint Medicine, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, Menarini International, Otsuka Pharmaceutical, Pfizer, Servier Pharmaceuticals LLC, and Syndax. Mauro Turrini reports honoraria from Johnson & Johnson, Astellas, and ItalFarmaco; fees for travel from Jazz Ph, AstraZeneca, and Johnson & Johnson; fees for professional activities from AstraZeneca and Astellas; and an unpaid role with the Regional Committee on Acute Leukemias. Calogero Vetro reports consulting fees from AbbVie and Jazz Pharmaceuticals. The other authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

