Screening and hit evaluation of a microbial metabolite library against the pathogenic Plasmodium falciparum and Toxoplasma gondii parasites
- PMID: 40840407
- PMCID: PMC12396409
- DOI: 10.1016/j.ijpddr.2025.100606
Screening and hit evaluation of a microbial metabolite library against the pathogenic Plasmodium falciparum and Toxoplasma gondii parasites
Abstract
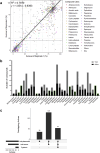
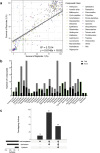
Frontline drug treatments for malaria are at risk of failing due to emerging resistance, meanwhile drugs used to treat toxoplasmosis have suboptimal efficacy and safety. As demonstrated by the success of clinically used antiparasitic drugs, the diverse structural complexity and biological activity of natural products holds great potential for drug discovery and development, to address the need for new compounds with novel mechanisms. Here we screened the BioAustralis Discovery Plates Series I library, a collection of 812 microbial natural product compounds including rare microbial metabolites, against Plasmodium falciparum erythrocytic stage and Toxoplasma gondii tachyzoite parasites. We identified 219 compounds that inhibited P. falciparum growth by at least 80 % at a concentration of 2 μg/mL (1-10 μM for >90 % of compounds), whilst 149 compounds demonstrated equivalent activity against T. gondii. The active compounds were assigned based on chemical structure to more than 50 compound classes. After triaging active compounds for those with low mammalian cytotoxicity, we defined the in vitro half maximal inhibitory concentration (IC50) of a selection of compounds against the parasites, identifying four compound groups with activity in the low nanomolar range. The macrocyclic lactone pladienolide B and cryptopleurine were found to be very potent against the parasites but also mammalian cells, warranting further structure-activity relationship investigation. Two groups, the monocyclic thiazole peptides, including micrococcin P1 and the thiocillins, and the pleuromutilins, exhibited both low antiparasitic IC50 and low cytotoxicity, highlighting their potential for further analysis. This study defines the activity of the BioAustralis Discovery Plates Series I against two apicomplexan parasites of significant global importance, providing potential new tools to study parasite biology and possible starting points for novel antiparasitic development.
Keywords: Drug discovery; Malaria; Natural products; Toxoplasmosis.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement Authors MRG, EYM, OR, DV, RO, SWP, EL, and DWW declare no financial or non-financial competing interests. Author SWP holds shares in Advanced Veterinary Therapeutics, Luoda Pharma and Neoculi, and has previously acted as a paid consultant for Zoetis, Boehringer Ingleheim, Elanco, Virbac and Ceva but declares no financial or non-financial competing interests.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Catechin gallate triggers metabolomic and lipidomic alteration in Toxoplasma gondii.Parasit Vectors. 2025 Jul 31;18(1):317. doi: 10.1186/s13071-025-06869-x. Parasit Vectors. 2025. PMID: 40745643 Free PMC article.
-
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission.Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. PMID: 24979199 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Amaratunga C., Lim P., Suon S., Sreng S., Mao S., Sopha C., Sam B., Dek D., Try V., Amato R., Blessborn D., Song L., Tullo G.S., Fay M.P., Anderson J.M., Tarning J., Fairhurst R.M. Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect. Dis. 2016;16(3):357–365. doi: 10.1016/S1473-3099(15)00487-9. - DOI - PMC - PubMed
-
- Aminake M.N., Schoof S., Sologub L., Leubner M., Kirschner M., Arndt H., Pradel G. Thiostrepton and derivatives exhibit antimalarial and gametocytocidal activity by dually targeting parasite proteasome and apicoplast. Antimicrob. Agents Chemother. 2011;55(4):1338–1348. doi: 10.1128/AAC.01096-10. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

