Prospective Multicenter Evaluation of [11C]Methionine PET/MRI Sensitivity Compared with MRI for Localizing Small Pituitary Neuroendocrine Tumor or Pituitary Adenoma in Cushing Disease
- PMID: 40841148
- PMCID: PMC12487729
- DOI: 10.2967/jnumed.124.269392
Prospective Multicenter Evaluation of [11C]Methionine PET/MRI Sensitivity Compared with MRI for Localizing Small Pituitary Neuroendocrine Tumor or Pituitary Adenoma in Cushing Disease
Abstract
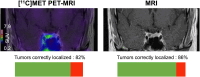
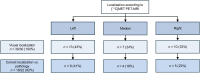
Functional small pituitary neuroendocrine tumors (PitNETs) of the corticotroph type lead to Cushing disease (CD), a condition associated with significant morbidity and increased mortality. Pituitary MRI is the primary imaging modality used to localize the tumor, but it is inconclusive in up to 30% of cases. Accumulating retrospective evidence suggests that [11C]methionine ([11C]MET) PET could address this diagnostic gap. This prospective study evaluates the sensitivity of [11C]MET PET/MRI compared with MRI for small PitNET localization in CD. Methods: This prospective multicenter study (ClinicalTrials.gov NCT03346954) included consecutive patients with biochemically confirmed de novo CD who underwent [11C]MET PET/MRI before surgery. Images were evaluated by experienced radiologists and nuclear medicine physicians. Their sensitivity to correctly localized PitNETs was calculated, with pathologic analysis of the surgical specimen used as a reference. Results: Thirty patients (73% women; mean age, 39.4 ± 12.7 y) underwent PET/MRI, and pathology confirmed PitNET in 22 patients (73%). [11C]MET PET/MRI correctly localized the tumor in 18 patients (82%; 95% CI, 0.60-0.95), whereas MRI correctly localized the tumor in 19 patients (86%; 95% CI, 0.65-0.97; P = 1.0). [11C]MET PET/MRI and MRI were concordant in 18 patients (82%), with the tumor correctly localized in 17 of 18 patients (94%). Among the 4 patients with discordant findings, MRI correctly localized the tumor in 2 cases for which PET was inaccurate; in 1 case, PET accurately identified the lesion, which was not visualized on MRI, and in the final case, both modalities failed to localize the tumor. Conclusion: This prospective study shows that [11C]MET PET/MRI has high sensitivity but does not differ significantly from 3-T MRI for the accurate localization of small PitNETs of the corticotroph type in patients with de novo CD. Future research could benefit from emerging imaging technologies and should focus on optimizing PET imaging protocols and exploring alternative radiotracers.
Keywords: PET/MRI; [11C]methionine; amino acid; brain; corticotroph.
© 2025 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

References
-
- Giuffrida G, Crisafulli S, Ferraù F, et al. Global Cushing’s disease epidemiology: a systematic review and meta-analysis of observational studies. J Endocrinol Invest. 2022;45:1235–1246. - PubMed
-
- Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr Pathol. 2022;33:6–26. - PubMed
-
- Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BMK, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;4:611–629. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
