Davanat-Mimetic Galactomannan and Its Sulfated Derivative: Structure and Antitumor Effects against Melanoma
- PMID: 40841319
- PMCID: PMC12421689
- DOI: 10.1021/acs.biomac.5c00290
Davanat-Mimetic Galactomannan and Its Sulfated Derivative: Structure and Antitumor Effects against Melanoma
Abstract
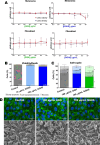
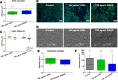
Melanoma is the most aggressive skin cancer, with a high metastatic potential and limited treatment options in advanced stages. Polysaccharides are promising antitumor agents, and therefore, this study investigated a galactomannan from guar gum hydrolysis (GGH) and its sulfated derivative (GGHS) for their antimelanoma and immunostimulatory effects. GGH shares structural similarity with DAVANAT, a galectin-1 ligand with anticolorectal cancer activity, while GGHS has anticoagulant properties, like heparin used in cancer patients. In vitro, 100 μg/mL GGH or GGHS inhibited melanoma cell invasion, increased adhesion, and reduced colony size, while GGHS also reduced proliferation. Both compounds bind galectin-3 and -1, but only GGH suppressed tumor progression in mice. Both treatments stimulated macrophage proinflammatory responses, including reactive oxygen species production and cytokine secretion. Although in vitro lymphocyte proliferation was not observed, CD3+ cells increased in the metastatic lungs. These results suggest GGH and GGHS as immunostimulatory agents, with GGH as potential melanoma adjuvant therapy.
Figures





References
-
- Chen Y., Sumardika I. W., Tomonobu N., Winarsa Ruma I. M., Kinoshita R., Kondo E., Inoue Y., Sato H., Yamauchi A., Murata H., Yamamoto K., Tomida S., Shien K., Yamamoto H., Soh J., Liu M., Futami J., Sasai K., Katayama H., Kubo M., Putranto E. W., Hibino T., Sun B., Nishibori M., Toyooka S., Sakaguchi M.. Melanoma Cell Adhesion Molecule Is the Driving Force behind the Dissemination of Melanoma upon S100A8/A9 Binding in the Original Skin Lesion. Cancer Lett. 2019;452:178–190. doi: 10.1016/j.canlet.2019.03.023. - DOI - PubMed
-
- Krishnan V., Bane S. M., Kawle P. D., Naresh K. N., Kalraiya R. D.. Altered Melanoma Cell Surface Glycosylation Mediates Organ Specific Adhesion and Metastasis via Lectin Receptors on the Lung Vascular Endothelium. Clin. Exp. Metastasis. 2005;22(1):11–24. doi: 10.1007/s10585-005-2036-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

