The bispecific innate cell engager AFM28 eliminates CD123+ leukemic stem and progenitor cells in AML and MDS
- PMID: 40841539
- PMCID: PMC12371032
- DOI: 10.1038/s41467-025-63069-y
The bispecific innate cell engager AFM28 eliminates CD123+ leukemic stem and progenitor cells in AML and MDS
Abstract
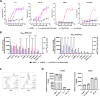
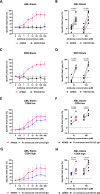
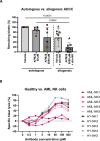
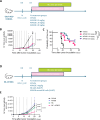
Strategies targeting leukemic stem and progenitor cells (LSPCs) are needed for durable remissions in acute myeloid leukemia (AML) and high-risk myelodysplastic neoplasms (MDS). While CD123 constitutes a promising target on LSPCs and leukemic blasts, previous CD123-targeting approaches showed limited efficacy and challenging safety profiles. Here, we describe the preclinical efficacy and safety of the bispecific CD123/CD16A innate cell engager "AFM28", demonstrating superior activity against AML and MDS patient-derived LSPCs and blasts in vitro compared to an Fc-enhanced CD123-targeting antibody, especially towards CD123low and/or CD64+ leukemic cells. AFM28 induces autologous anti-leukemic activity in fresh AML whole blood cultures, demonstrating its potential to enhance NK cell function from AML patients. Responsiveness can be further enhanced by allogeneic NK cell addition. Anti-leukemic activity of AFM28 is confirmed in xenograft mouse models. In addition, AFM28 is well tolerated and demonstrates pharmacodynamic activity in cynomolgus monkeys. Altogether, our results indicate that AFM28 has the potential to reduce relapse-inducing residual disease and promote long-term remissions for patients with AML and MDS with a favorable safety profile.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: This study was supported by funding from Affimed GmbH. J.P. is an employee of Affimed GmbH. J.J.S., S.S., U.R., J.M.E., J.K., A.L.G., T.R., T.M., I.K., SKnackmuss, C.M., and J.E. were employees of Affimed at the time of writing and execution of this study. D.N. has received research funding from Affimed, Tolero Pharma, Pharmaxis and Apogenix, received funding for a clinical trial from AbbVie and received honoraria from Celgene and Takeda. The remaining authors declare no competing interests.
Figures


References
-
- Bonnet, D. & Dick, J. E. Human acute myeloid Leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med.3, 730–737 (1997). - PubMed
-
- Nilsson, L. et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood96, 2012–2021 (2000). - PubMed
-
- National Cancer Institute. Cancer Stat Facts: Leukemia – Acute Myeloid Leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html (2022).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

