Prefrontal cortex astrocytes modulate distinct neuronal populations to control anxiety-like behavior
- PMID: 40841557
- PMCID: PMC12371101
- DOI: 10.1038/s41467-025-63131-9
Prefrontal cortex astrocytes modulate distinct neuronal populations to control anxiety-like behavior
Abstract
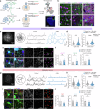
Accumulating evidence has supported diverse regulatory functions of astrocytes in different neural circuits as well as various aspects of complex behaviors. However, little is known about how astrocytes regulate different neuronal subpopulations that are linked to specific behavioral aspects within a single brain region. Here, we show that astrocytes in the medial prefrontal cortex (mPFC) encode anxiogenic environmental cues in freely behaving mice. Silencing mPFC astrocyte Ca2+ signaling heightens anxiety-like behavior and triggers opposing functional responses in excitatory and inhibitory neurons. Moreover, neuronal subpopulations tuned to anxiety-like behavior are differentially modulated by mPFC astrocytes at single cell and network levels. Using cell type-specific proximity biotinylation approaches, we identified significant intracellular and intercellular proteomic alterations in mPFC astrocytes and at the astrocyte-neuron interface associated with anxiety. Collectively, our findings uncover mechanisms underpinning the heterogenous astrocyte-neuron interaction that is behaviorally relevant and offer critical insights into the pathophysiology of emotional disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Grants and funding
- P30 DA018310/DA/NIDA NIH HHS/United States
- BRFSG-2021-12/Brain Research Foundation (BRF)
- R01 MH132556/MH/NIMH NIH HHS/United States
- DP2 NS136871/NS/NINDS NIH HHS/United States
- 2021-08-025/Whitehall Foundation (Whitehall Foundation, Inc.)
- P30DA018310/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- 30748/Brain and Behavior Research Foundation (Brain & Behavior Research Foundation)
- DP2 NS136871D/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

