Improving the accuracy of Respiratory Syncytial Virus (RSV) incidence estimates among hospitalised adults in Bristol, UK
- PMID: 40841602
- PMCID: PMC12369217
- DOI: 10.1186/s12879-025-11292-9
Improving the accuracy of Respiratory Syncytial Virus (RSV) incidence estimates among hospitalised adults in Bristol, UK
Abstract
Background: The burden of Respiratory Syncytial Virus (RSV) infection in adults is of interest in the context of recently-licensed vaccines. However, burden estimates are affected by test error associated with the testing platform, and number and type of samples tested.
Methods: We conducted a prospective cohort study of adults with acute lower respiratory tract disease (aLRTD) hospitalised in Bristol, UK, from April 2022-March 2023. RSV was detected by RT-PCR both by routine standard-of-care (SOC) testing, and by testing of additional nasopharyngeal swabs, saliva and sputum samples from a patient subset. Latent class analysis was used to quantify and adjust for test error rates, including effects of multiple testing. RSV test-positivity rates are reported, and after adjustment for test error, are used to calculate adult population incidence/1000 person-years.
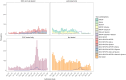
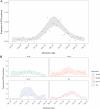
Results: 6906/11445 aLRTD cases (60%) were tested and 251 were positive (3.6%; 251/6906). Test-positivity peaked in December (95%CI 7.9-12.7%). Among cases, 43% had pneumonia, 55% had non-pneumonic infection, 59% chronic respiratory disease exacerbations, and 16% heart failure. Test-positivity was highest in 75-84-year-olds, and 30-day mortality was highest in ≥ 75-year-olds (7.1%; 9/127). Due to low positivity-rates and imperfect specificity (0.98-1.00), test-positivity (3.6%) overestimated inferred true prevalence (2.3%). After adjustment for test error, we estimate overall adult population incidence/1000-person-years to be 0.33 (0.21-0.49), and 2.02 (1.10-3.06) in ≥ 75-year-olds.
Conclusions: RSV contributes significantly to hospitalised adult aLRTD, particularly among the elderly. The implementation of effective RSV vaccines could reduce morbidity, mortality and associated costs of disease. Adult RSV burden accuracy is improved by adjustment for test characteristics due to the impact of imperfect specificity when positivity-rates are low, and this is particularly important for out-of-season estimates. Multiple samples can improve burden estimation accuracy only when tests have near-perfect specificity.
Keywords: Cardiac failure; Lower respiratory tract infection; Pneumonia; RSV; Test error.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This prospective observational cohort study of adults admitted to two large university hospitals (Southmead, and Bristol Royal Infirmary) in Bristol, UK, was conducted in accordance with the International Conference for Harmonisation of Good Clinical Practice (ICCH GCP), the UK Policy Framework for Health and Social Care Research, and the Declaration of Helsinki, and was approved by the East of England—Essex Research Ethics Committee, Health Research Authority reference 20/EE/0157, ISRCTN: 17354061. Informed consent was obtained from patients with capacity, and declarations for participation from consultees for individuals lacking capacity. If it was not practicable to approach individuals for consent, data were included using approval from the Clinical Advisory Group under Sect. 251 of the 2006 NHS Act. Clinical trial number: not applicable. Competing interests: CH is Principal Investigator of the AvonCAP study which is an investigator-led University of Bristol study funded by Pfizer and has previously received support from the NIHR in an Academic Clinical Fellowship. JO is a Co-Investigator on the AvonCAP Study. LD is further supported by UKRI through the JUNIPER consortium (grant number MR/V038613/1), MRC (grant number MC/PC/19067), EPSRC (EP/V051555/1 and The Alan Turing Institute, grant EP/N510129/1). AF was a member of the Joint Committee on Vaccination and Immunization (JCVI) until January 2024 and remains a member of the JCVI RSV subcommittee. In addition to receiving funding from Pfizer as Chief Investigator of this study, he leads another project investigating transmission of respiratory bacteria in families jointly funded by Pfizer and the Gates Foundation and is an investigator in trials of COVID-19 vaccines including ChAdOx1nCOV-19, Janssen, Sanofi and Valneva vaccines. NM is supported by the National Institute for Health and Care Research Bristol Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. This study was conducted as a collaboration between The University of Bristol (study sponsor) and Pfizer (study funder).
Figures



References
-
- Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J. 2011. 10.1097/INF.0b013e3182184ae7. - PubMed
-
- Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015. 10.1007/s40266-015-0258-9. - PubMed
-
- Branche AR, Saiman L, Walsh EE, Falsey AR, Sieling WD, Greendyke W, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis. 2022. 10.1093/cid/ciab595. - PubMed
-
- Nguyen-Van-tam JS, O’leary M, Martin ET, Heijnen E, Callendret B, Fleischhackl R, Comeaux C, Tran TMP, Weber K. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. 2022;31(166):220105. 10.1183/16000617.0105-2022. PMID: 36384703; PMCID: PMC9724807. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

