Locus coeruleus tonic upregulation increases selectivity to inconspicuous auditory information in autistic compared to non-autistic individuals: a combined pupillometry and electroencephalography study
- PMID: 40841687
- PMCID: PMC12372287
- DOI: 10.1186/s13229-025-00678-w
Locus coeruleus tonic upregulation increases selectivity to inconspicuous auditory information in autistic compared to non-autistic individuals: a combined pupillometry and electroencephalography study
Abstract
Background: Sensory processing requires selectivity to salient sensory input. Many autistic individuals report different sensory processing, which has been associated with altered sensory selectivity. The locus-coeruleus norepinephrine (LC-NE) system modulates the neuronal gain of sensory input, which represents a neurophysiological mechanism of sensory selectivity. In autistic individuals, we hypothesized that LC-NE tonic upregulation reduces sensory selectivity and underlies different sensory processing.
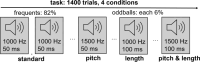
Methods: Autistic (n = 139) and non-autistic (n = 98) individuals were assessed during a passive auditory oddball task with pupillometry and electroencephalography. For every trial, a baseline pupil size (BPS) assessed LC-NE tonic activity that coincides with current arousal, while a stimulus-evoked pupillary response (SEPR) assessed LC-NE phasic activity that estimated sensory selectivity. Electroencephalography assessed amplitudes of mismatch negativity (MMN-amp) that estimated pre-attentive change detection as a brain-activity readout of sensory selectivity. Measures were modeled between groups within the task by combining Frequentist and Bayesian approaches.
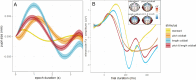
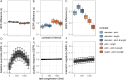
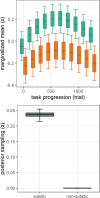
Results: Across groups, higher BPS was associated with more negative MMN-amp to standards and oddballs. A more negative MMN-amp to standards was associated with a higher SEPR to standards. Controlling for these associations, autistic versus non-autistic individuals showed a higher SEPR in response to standards. In addition, a positive association of BPS and SEPR to standards was specific to autistic individuals. With task progression, autistic versus non-autistic individuals showed a higher initial increase and subsequently steeper decrease of BPS. This was supported by Bayesian posterior distribution estimates.
Limitations: A short trial duration required concatenating trials to epochs and applying a linear-time invariant filter to capture the slow pupil changes. Without an LC-NE manipulation, we cannot rule out that pupil changes are evoked by other cortical pathways than the LC-NE.
Conclusions: Across groups, LC-NE tonic upregulation is emphasized as a general mechanism that un-specifically increases pre-attentive change detection to all sensory stimuli, which then increases sensory selectivity to frequent stimuli. In autistic individuals, different sensory processing is characterized by increased sensory selectivity to frequent stimuli. This is likely caused by an LC-NE tonic upregulation. It associates autistic sensory processing with increased arousal upregulation that increases sensory selectivity to inconspicuous auditory information.
Keywords: Arousal; Auditory oddball paradigm; Autism spectrum condition; Mismatch negativity; Predictive coding; Pupillometry.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: All participants (where appropriate) and their parent/legal guardian provided written informed consent. Ethical approval for this study was obtained through ethics committees at each site (King’s College London—London Queen Square Health Research Authority Research Ethics Committee: 13/LO/1156; Autism Research Centre, University of Cambridge—London Queen Square Health Research Authority Research Ethics Committee: 13/LO/1156; Radboud University Medical Centre—Quality and Safety Committee on Research Involving Human Subjects Arnhem-Nijmegen: 2013/455, University Medical Centre Utrecht—- Quality and Safety Committee on Research Involving Human Subjects Arnhem-Nijmegen: 2013/455; Central Insitute of Mental Health—University Medical Mannheim, Medical Ethics Commission II: 2014-540N-MA; Universita Campus Bio-Medica De Roma—Medical Ethics Committee: 18/14 PAR ComET CBM; Karolinska Intitute – Central Ethical Review Board: 32–2010). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

