Morphological heterogeneities in prostate cancer bone metastases are related to molecular subtypes and prognosis
- PMID: 40841830
- PMCID: PMC12370827
- DOI: 10.1007/s10585-025-10365-y
Morphological heterogeneities in prostate cancer bone metastases are related to molecular subtypes and prognosis
Abstract
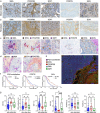
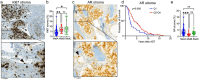
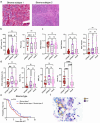
We previously identified three molecular subtypes of prostate cancer (PC) bone metastases, MetA-C, with MetB linked to poor prognosis after androgen deprivation therapy (ADT). This study analyzed epithelial and stromal markers using immunohistochemistry, focusing on their relationship to MetA-C subtypes, spatial heterogeneities, and clinical outcomes after ADT. High tumor proliferation and low PSA expression were associated with MetB and poor outcomes after ADT. Most metastases contained tumor epithelial subclones with different morphologies. In the metastasis stroma, blood vessels and fibroblast-like cells expressed smooth muscle actin (SMA), platelet-derived growth factor β, stroma-derived factor 1 (SDF1), periostin (POSTN), and decorin (DCN). Compared to each other, MetB metastases had higher SMA and ERG + endothelial cell densities, while MetA cases showed higher SDF1 and DCN levels. Accordingly, high POSTN and ERG + densities were associated with poor outcomes after ADT, whereas high DCN indicated favorable prognosis. Low levels of AR-positive stromal cells were linked to poor outcomes. Macrophage and T-lymphocyte densities showed no significant associations with metastases subtypes or outcome. Two stroma subtypes were identified: subtype 1 with higher bone content, lower vessel density, MetA-enrichment and better prognosis compared to subtype 2 that exhibited higher tumor proliferation and lower PSA expression. Most metastases contained regions of both stroma subtypes.
Keywords: Androgen deprivation therapy; Bone metastasis stroma; Metastases morphology; Metastatic stroma; Prostate cancer metastases; Prostate cancer molecular subtypes; Stromal markers; Tumor heterogeneity; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: A.B., E.T., and P.W. have a pending patent application related to the metastasis subtypes MetA-C and to Ki67 and PSA immunostaining as surrogate markers for the molecular metastasis subtypes (‘Methods for diagnosis and prognosis of prostate cancer’, EP2020/054681).
Figures



References
-
- Cornford P et al (2021) EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate Cancer. Eur Urol 79(2):263–282 - PubMed
-
- Crnalic S et al (2010) Nuclear androgen receptor staining in bone metastases is related to a poor outcome in prostate cancer patients. Endocr-Relat Cancer 17(4):885–895 - PubMed
-
- Ohlson N et al (2005) Cell proliferation and apoptosis in prostate tumors and adjacent non-malignant prostate tissue in patients at different time-points after castration treatment. Prostate 62(4):307–315 - PubMed
-
- Iglesias-Gato D et al (2018) The proteome of prostate cancer bone metastasis reveals heterogeneity with prognostic implications. Clin Cancer Res 24(21):5433–5444 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

