A new era in cancer therapy: targeting the Proteasome-Bcl-2 axis
- PMID: 40841912
- PMCID: PMC12369272
- DOI: 10.1186/s13046-025-03505-5
A new era in cancer therapy: targeting the Proteasome-Bcl-2 axis
Abstract
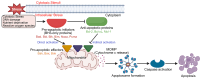
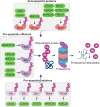
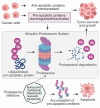
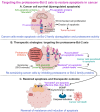
The B-cell lymphoma-2 (Bcl-2) family proteins, key regulators of apoptosis, are frequently dysregulated in cancer, tipping the balance of cell survival and apoptosis in favor of survival. The ubiquitin-proteasome system (UPS) is a critical cellular machinery that controls the Bcl-2 levels through regulation of protein stability. This review delves into the intricate interplay between the proteasome and Bcl-2 family members, exploring how proteasome-mediated degradation impacts cell survival and proliferation to influence cancer progression. We discuss the therapeutic potential of targeting the proteasome-Bcl-2 axis, including the use of proteasome inhibitors as anticancer agents. We examine their mechanisms of action, clinical efficacy, and limitations while exploring emerging strategies to overcome these challenges.
Keywords: Apoptosis; Bcl-2 family proteins; Cancer; Proteasome inhibitors; Therapeutic potential; Ubiquitin-proteasome system (UPS); Ubiquitination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

