Early use of albumin may increase the risk of sepsis-associated acute kidney injury in sepsis patients: a target trial emulation
- PMID: 40841985
- PMCID: PMC12369241
- DOI: 10.1186/s40779-025-00641-z
Early use of albumin may increase the risk of sepsis-associated acute kidney injury in sepsis patients: a target trial emulation
Abstract
Background: Most sepsis patients develop sepsis-associated acute kidney injury (SA-AKI), which poses a significant threat to survival and lacks specific treatment. To date, there are no published randomized controlled trials that have established a link between albumin use and SA-AKI development in sepsis. Therefore, it is unclear whether albumin use may influence the risk of SA-AKI.
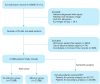
Methods: The present study employed a target trial emulation using observational data to track adult sepsis patients initially admitted to the intensive care unit at Beth Israel Deaconess Medical Center, Boston, Massachusetts, for a period of 7 d from 2008 to 2022. Immortal time bias was controlled using the clone-censor-weight (CCW) method, along with a new-user design to address current user bias. The exposure variable was the early administration of albumin following the onset of sepsis. Based on albumin use, patients were classified into two groups: the albumin group (n = 27,088) and the no albumin group (n = 27,088). The primary outcome was the development of SA-AKI, and the secondary outcome was 7-day all-cause mortality. The primary outcome was analyzed using competing risk analyses. Furthermore, sensitivity and subgroup analyses were also performed.
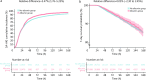
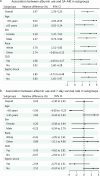
Results: Among the 27,088 patients analyzed, albumin administration was associated with a significantly higher SA-AKI risk (relative difference = 3.47%, 95% CI 1.76-5.23) compared to non-administration. There was no clinically meaningful difference in 7-day survival (relative difference = 0.05%, 95% CI -2.30 to 2.45). Sensitivity analyses consistently supported these results. All these analyses were conducted on data that were collected after CCW.
Conclusions: Early albumin administration may increase the risk of SA-AKI in sepsis patients without conferring a short-term survival benefit. These results underscore the need for a rigorous risk-benefit assessment when incorporating albumin into sepsis resuscitation protocols and highlight the need for further clinical validation. However, it is important to exercise caution when interpreting the conclusions of this study, given its exploratory and preliminary nature.
Keywords: Albumin; Clone-censor-weight (CCW); Sepsis-associated acute kidney injury (SA-AKI); Target trial emulation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Medical Information Mart for Intensive Care IV (MIMIC-IV) database was supported by grants from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH) under award numbers R01-EB001659 (2003–2013) and R01-EB017205 (2014–2018), and approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Iba T, Helms J, Maier CL, Levi M, Scarlatescu E, Levy JH. The role of thromboinflammation in acute kidney injury among patients with septic coagulopathy. J Thromb Haemost. 2024;22(6):1530–40. - PubMed
-
- White KC, Serpa-Neto A, Hurford R, Clement P, Laupland KB, See E, et al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med. 2023;49(9):1079–89. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

