Sirt4 Deficiency Promotes Cardiomyocyte Proliferation and Cardiac Repair
- PMID: 40842073
- PMCID: PMC12370541
- DOI: 10.1111/jcmm.70741
Sirt4 Deficiency Promotes Cardiomyocyte Proliferation and Cardiac Repair
Abstract
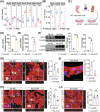
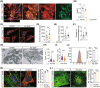
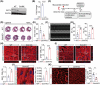
The mammalian heart exhibits transient but remarkable regenerative capacity during the early postnatal period, after which most cardiomyocytes exit the cell cycle. While the sirtuin family is well-established as regulators of cell cycle progression, its specific role in cardiomyocyte proliferation and cardiac regeneration remains unclear. In this study, we found that Sirt4 expression increased during postnatal heart development. Adenovirus-mediated Sirt4 overexpression in vitro inhibited cardiomyocyte proliferation by inducing oxidative DNA damage. Moreover, cardiomyocyte-specific Sirt4 overexpression in vivo suppressed cardiomyocyte proliferation and impaired neonatal heart regeneration. Using Sirt4-knockout mice, we found that Sirt4 deficiency promoted cardiomyocyte proliferation and extended the heart regeneration window. Furthermore, Sirt4 deficiency improved cardiac function and reduced myocardial fibrosis after ischaemia-reperfusion injury in adult mice. These findings establish Sirt4 as a critical regulator of cardiomyocyte proliferation and cardiac repair, suggesting that targeted Sirt4 inhibition may represent a promising therapeutic strategy for ischaemic heart diseases.
Keywords: Sirt4; cardiomyocyte proliferation; heart regeneration; oxidative DNA damage.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Doiron J. E. and Lefer D. J., “Leveraging Adipocyte‐Cardiomyocyte Signaling to Treat Ischemic Heart Failure,” Circulation Research 131 (2022): 165–167. - PubMed
MeSH terms
Substances
Grants and funding
- 2022YFA1104503/National Key Research and Development Project of China
- 82300325/National Natural Science Foundation of China
- 82300448/National Natural Science Foundation of China
- 82370304/National Natural Science Foundation of China
- 82425006/National Natural Science Foundation of China
- 2024-FZX04/Henan Cardiovascular Disease Center (Central China Subcenter of National Center for Cardiovascular Diseases)
- 2021-I2M-1-016/CAMS Innovation Fund for Medical Sciences
- 2023-PT310-03/non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences
- 2023-GSP-ZD-2-01/National High Level Hospital Clinical Research Funding
LinkOut - more resources
Full Text Sources

