Astrocyte alterations in α-synucleinopathies
- PMID: 40842565
- PMCID: PMC12364820
- DOI: 10.3389/fncel.2025.1650326
Astrocyte alterations in α-synucleinopathies
Abstract
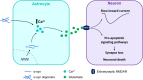
Despite long being considered to be passive and supportive cells, in the last decades astrocytes have arisen as key regulators of neuronal excitability, synaptic transmission and plasticity. Since the discovery of the tripartite synapse, accumulating evidence suggests that astrocytes are involved in the pathogenesis of neurodegenerative diseases, including α-synucleinopathies. Here we will discuss recent evidence showing that astrocytes express endogenous α-synuclein and the implications of this protein in astrocyte cellular processes. Furthermore, we review how the expression of pathological forms of this protein in astrocytes leads to aberrant cytosolic Ca2+ activity in these cells and to alterations in gliotransmission and pathology progression.
Keywords: astrocytes; gliotransmission; tripartite synapse; α-synuclein; α-synucleinopathies.
Copyright © 2025 Rodríguez-Castañeda, Campos-Ríos, Lamas and Covelo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

