Spontaneous chiral symmetry breaking in a single crystal
- PMID: 40842696
- PMCID: PMC12362298
- DOI: 10.1039/d5sc02623g
Spontaneous chiral symmetry breaking in a single crystal
Abstract
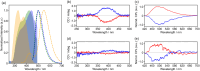
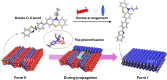
Chiral symmetry breaking (CSB) under nonequilibrium open conditions is a ubiquitous phenomenon in the universe, whereas molecular-level CSB is limited. Only the preferential enrichment and Viedma ripening that occur during the crystallization process from a solution are known. Herein, we discovered the third category of CSB, which is complete CSB within a single crystal. A racemic crystal of 3-(4-(benzo[d]thiazol-2-yl)phenyl)-10-propyl-10H-phenothiazine, a phenothiazine derivative with dynamic chirality, undergoes a single-crystal-to-single-crystal structural transition to a chiral crystal. Furthermore, the chirality after the transition is able to be controlled by solid-seeding of a chiral crystal. The resulting chiral single crystals exhibited circularly polarized luminescence (CPL) properties (g lum = 8.9 × 10-4). This discovery provides a simple model of CSB and stimuli-responsive materials involving the CSB phenomenon.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Klevansky S. P. Rev. Mod. Phys. 1992;64:649. doi: 10.1103/RevModPhys.64.649. - DOI
-
- Pasteur L. Ann. Chim. Phys. 1848;24:442–459.
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

