Wharton's Jelly Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Intervertebral Disc Degeneration Under Inflammatory Stress in an In Vitro 3D Culture System
- PMID: 40842947
- PMCID: PMC12366443
- DOI: 10.1002/jsp2.70106
Wharton's Jelly Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Intervertebral Disc Degeneration Under Inflammatory Stress in an In Vitro 3D Culture System
Abstract
This study explores the therapeutic potential of extracellular vesicles (EVs) derived from Wharton's Jelly mesenchymal stem cells in an in vitro 3D model of intervertebral disc degeneration under inflammatory stress. The treatment with WJ-MSC-EVs enhanced nucleus pulposus cell proliferation, viability, and extracellular matrix synthesis while reducing oxidative stress and catabolic gene expression. These results support the promise of WJ-MSC-EVs as a novel, cell-free strategy for disc regeneration in inflammatory conditions.
Background: Extracellular vesicles (EVs) derived from mesenchymal stromal cells (MSCs) are emerging as a promising cell‐free strategy for intervertebral disc degeneration (IDD) treatment. This study aimed to evaluate the anabolic effect of Wharton's Jelly MSC (WJ‐MSC)‐derived EVs on degenerative human nucleus pulposus cells (hNPCs) under in vitro inflammation using a 3D culture model.
Methods: Following isolation, hNPCs (n = 10) were encapsulated in alginate beads and treated with 10, 50, and 100 μg/mL of WJ‐MSC‐EVs after preincubation with 10 ng/mL interleukin (IL)‐1β. Cell proliferation, viability, nitrite production, and glycosaminoglycan (GAG) content were assessed. Histological analyses evaluated extracellular matrix (ECM) production. Phenotypic (SOX9, KRT19), catabolic (MMP1, MMP13, ADAMTS5, IL6, NOS2), and anabolic (ACAN) ECM markers were analyzed by RT‐qPCR.
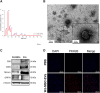
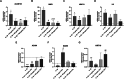
Results: WJ‐MSC‐EVs significantly promoted hNPC proliferation at all concentrations, with 10 μg/mL effectively counteracting IL‐1β catabolic effects. Live/dead staining showed reduced cell death in EV‐treated hNPCs compared to the IL‐1β‐only group. Nitrite production decreased after 7 days with 10 μg/mL WJ‐EVs, supported by reduced NOS2 expression. GAG content increased dose‐dependently, as confirmed by Alcian blue staining. WJ‐EVs positively modulated anabolic (ACAN, KRT19, SOX9), catabolic (ADAMTS5, MMP1, MMP13), and inflammatory (IL6) gene expression levels.
Conclusion: WJ‐MSC‐derived EVs demonstrate potential as a cell‐free therapeutic approach for IDD by enhancing hNPC growth, mitigating ECM degradation, and reducing oxidative stress‐related IDD progression. These findings warrant further investigation into the use of WJ‐EVs for IDD treatment.
Keywords: exosome; extracellular vesicles; intervertebral disc; intervertebral disc degeneration; intervertebral disc regeneration; low back pain; mesenchymal stromal cells.
© 2025 The Author(s). JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Figures




References
LinkOut - more resources
Full Text Sources

