Loss of Cullin 5 in myeloid cells protects against autoimmune neuroinflammation
- PMID: 40842987
- PMCID: PMC12366467
- DOI: 10.3389/fimmu.2025.1611818
Loss of Cullin 5 in myeloid cells protects against autoimmune neuroinflammation
Abstract
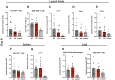
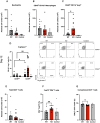
Autoimmune neuroinflammation occurs when an individual's immune cells attack the brain, spinal cord or peripheral nerves. Several Suppressor of Cytokine Signaling (SOCS) proteins have been shown to limit pro-inflammatory signaling pathways in myeloid cells and prevent neuroinflammation. They rely on several mechanisms to accomplish this. Their SH2 domain allows them to bind phosphorylated tyrosine residues on surface receptors to prevent downstream signaling while their C-terminal SOCS domain can promote their assembly with Cullin5 (CUL5) to degrade signaling proteins. To date, the role of CUL5 in myeloid-cell-mediated function is poorly understood. Here we show that loss of Cul5 in myeloid cells resulted in reduced neuroinflammation and attenuated progression of Experimental Autoimmune Encephalomyelitis (EAE). Although peripheral CD4+ T cell activation was not overtly affected, Cul5-deficient macrophages in the Central Nervous System (CNS) demonstrated a significant shift toward an anti-inflammatory phenotype, characterized by increased expression of Arginase 1. This correlated with an enhanced frequency of FoxP3+ regulatory T cells. In contrast to what would be predicted if CUL5 and SOCS proteins work together to degrade pro-inflammatory cytokine signaling, Cul5 deletion in myeloid cells selectively enhanced IL-4-mediated Arginase 1 expression. These findings identify CUL5 as an unanticipated pro-inflammatory mediator during neuroinflammation and reveal its potential as a therapeutic target for autoimmune diseases.
Keywords: Cullin 5; macrophage polarization; macrophages; neuroinflammation; ubiquitin ligase (E3).
Copyright © 2025 Ortega-Burgos, Dar, Tomishima, Guha, Brien, Porter, Bennett and Oliver.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Verberk SGS, van der Zande HJP, Baardman J, de Goede KE, Harber KJ, Keuning ED, et al. Myeloid ATP citrate lyase regulates macrophage inflammatory responses in vitro without altering inflammatory disease outcomes. Front Immunol. (2021) 12:669920. doi: 10.3389/fimmu.2021.669920, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

