Interactions between tumor microenvironment and resistance to transarterial and systemic treatments for HCC
- PMID: 40843361
- PMCID: PMC12366493
- DOI: 10.20517/cdr.2024.212
Interactions between tumor microenvironment and resistance to transarterial and systemic treatments for HCC
Abstract
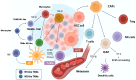
Hepatocellular carcinoma (HCC) is a malignant tumor originating from hepatocytes, often developing against a backdrop of chronic inflammation and liver fibrosis. The primary risk factor for HCC is cirrhosis, and early detection is crucial for improving outcomes. Despite advances in treatment, the prognosis remains poor, with a 5-year survival rate of approximately 15%-38%. Growing evidence highlights the critical role of the tumor microenvironment (TME) in modulating tumor initiation, growth, progression, and, in some cases, suppression. The TME is a complex ecosystem composed of immune cells, cancer-associated fibroblasts, extracellular matrix components, and other factors such as growth factors and cytokines. By shaping tumor cell behavior, the TME facilitates immune evasion and contributes to resistance to treatment. Tumor-associated immune cells, including regulatory T cells, myeloid-derived suppressor cells, and tumor-associated macrophages, contribute to immune suppression and progression. On the other hand, immune activation via immune checkpoint inhibition has shown promise in improving outcomes, especially when combined with other treatments such as transarterial chemoembolization (TACE), selective internal radiation therapy (SIRT), and systemic therapies. Studies have demonstrated the potential of targeting the TME to enhance treatment efficacy, with immune modulation emerging as a key therapeutic strategy. This review explores the complex interactions within the TME in HCC, highlighting its role in therapy resistance and immune evasion. It also discusses current therapeutic approaches to target the TME to improve clinical outcomes in HCC patients.
Keywords: Hepatocellular carcinoma; drug resistance; extracellular matrix; immune checkpoint inhibitors; immune evasion; selective internal radiation therapy; systemic therapy; transarterial chemoembolization.
© The Author(s) 2025.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
