Formation of neutrophil extracellular traps in the early stages exacerbate the healing process by regulating macrophage polarization in Achilles tendon-bone injury
- PMID: 40843411
- PMCID: PMC12365468
- DOI: 10.1177/20417314251348038
Formation of neutrophil extracellular traps in the early stages exacerbate the healing process by regulating macrophage polarization in Achilles tendon-bone injury
Abstract
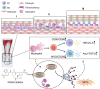
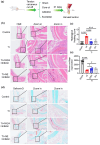
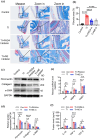
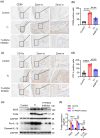
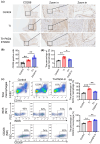
The influence of neutrophils and of neutrophil extracellular traps (NETs) on post-traumatic tendon-to-bone healing was studied in a murine model. The impact of neutrophil infiltration on macrophage polarization and peritendinous fibrosis in early-stage Achilles tendon injury is reported. Mice underwent Achilles tendon-bone injury and divided into four groups: sham operation, tendon injury (TI) treated with acetylcellulose (vehicle control), TI treated with a Protein arginine deiminase-4 (PAD4) inhibitor GSK484, and TI treated with a neutrophil elastase inhibitor Sivelestat. Each group was monitored for 21 days. Post-traumatic neutrophil infiltration and NET formation were assessed using flow cytometry and immunofluorescence. Immunohistochemistry, Western blot, and qPCR were used to evaluate macrophage polarization. Peritendinous fibrosis was assessed using Masson staining and Western blot. Neutrophil infiltration and NET formation increased significantly in the tendon following injury. A significant increase in M1-related markers and a decrease in M2-related markers were associated with NET formation. NET Inhibition using GSK484 or sivelestat reduced M1 markers and increased M2 markers. Furthermore, NET inhibition during the early stage suppressed peritendinous fibrosis and reduced inflammation during the healing process. In co-culture experiments, NETs induced proinflammatory cytokine secretion and upregulated M1 markers in bone marrow-derived macrophages while downregulating M2 markers. nlsNETs promote early-phase tendon-bone injury by inducing M1 macrophage polarization and peritendinous fibrosis. Targeting NETs during the initial phase of tendon injury could potentially facilitate the healing process.
Keywords: fibrosis; macrophages; neutrophil extracellular traps; neutrophils; tendon-bone injury.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
Leptin Enhances M1 Macrophage Polarization and Impairs Tendon-Bone Healing in Rotator Cuff Repair: A Rat Model.Clin Orthop Relat Res. 2025 May 1;483(5):939-951. doi: 10.1097/CORR.0000000000003428. Epub 2025 Feb 19. Clin Orthop Relat Res. 2025. PMID: 39982019
-
Mesenchymal stem cell transplantation alleviated TBI-induced lung injury by inhibiting PAD4-dependent NET formation.J Adv Res. 2025 Jun 14:S2090-1232(25)00438-2. doi: 10.1016/j.jare.2025.06.029. Online ahead of print. J Adv Res. 2025. PMID: 40523519
-
Intestinal neutrophil extracellular traps promote gut barrier damage exacerbating endotoxaemia, systemic inflammation and progression of diabetic retinopathy in type 2 diabetes.Diabetologia. 2025 Apr;68(4):866-889. doi: 10.1007/s00125-024-06349-4. Epub 2025 Jan 28. Diabetologia. 2025. PMID: 39875729 Free PMC article.
-
[Distribution characteristics of polymorphonuclear neutrophil pulmonary infiltration and the mechanism of neutrophil elastase in promoting lung injury in the early stages of severe burns].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2025 May;37(5):431-437. doi: 10.3760/cma.j.cn121430-20240816-00704. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2025. PMID: 40819943 Chinese.
-
Exploring the involvement of serine proteases in neutrophil extracellular traps: a review of mechanisms and implications.Cell Death Dis. 2025 Jul 18;16(1):535. doi: 10.1038/s41419-025-07857-w. Cell Death Dis. 2025. PMID: 40681487 Free PMC article. Review.
References
-
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018; 233: 6425–6440. - PubMed
-
- Sunwoo JY, Eliasberg CD, Carballo CB, et al. The role of the macrophage in tendinopathy and tendon healing. J Orthop Res 2020; 38: 1666–1675. - PubMed
LinkOut - more resources
Full Text Sources

