Generation of human induced pluripotent stem cell lines from patients with FGFR2-linked syndromic craniosynostosis
- PMID: 40843495
- PMCID: PMC12486208
- DOI: 10.1242/dmm.052123
Generation of human induced pluripotent stem cell lines from patients with FGFR2-linked syndromic craniosynostosis
Abstract
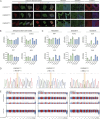
Craniosynostosis is a multigenic congenital condition in which one or more calvarial sutures have prematurely fused during the development of the fetus. Pathogenic variants in FGFR2 are associated with the development of syndromic craniosynostosis, such as Crouzon, Apert and Pfeifer syndromes. Investigation of FGFR2-linked craniosynostosis is hindered by the lack of appropriate in vitro models. Patient-derived human induced pluripotent stem cell (hiPSC) in vitro disease models provide the opportunity to investigate the disease, identify molecular targets for pharmaceutical treatments, and enable the generation of autologous pluripotent stem cell catalogues. Here, we report three patient-derived hiPSC lines carrying the C342Y, S252W or E565G FGFR2 pathogenic variant. The patient hiPSC lines express characteristic pluripotency markers and display distinct phosphorylation profiles under unstimulated conditions. FGFR2C342Y showed autophosphorylation in the absence of bFGF ligand, although downstream docking proteins PLCγ and FRS2α were not phosphorylated. FGFR2S252W and FGFR2E565G hiPSCs showed increased phosphorylation of docking proteins PLCγ and FRS2α, whereas FGFR2 was not phosphorylated. These patient hiPSC lines provide molecular and cellular options to investigate FGFR2-linked craniosynostosis in the patient-specific genomic context and develop therapeutic modalities.
Keywords: Apert; Craniosynostosis; Crouzon; FGFR2; Pfeiffer; hiPSC.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

