Mechanisms of GLP-1 in Modulating Craving and Addiction: Neurobiological and Translational Insights
- PMID: 40843757
- PMCID: PMC12372146
- DOI: 10.3390/medsci13030136
Mechanisms of GLP-1 in Modulating Craving and Addiction: Neurobiological and Translational Insights
Abstract
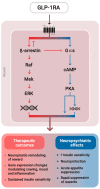
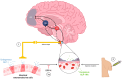
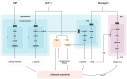
Substance use disorders (SUDs) remain a major public health challenge, with existing pharmacotherapies offering limited long-term efficacy. Traditional treatments focus on dopaminergic systems but often overlook the complex interplay between metabolic signals, neuroplasticity, and conditioned behaviors that perpetuate addiction. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), originally developed for type 2 diabetes and obesity, have recently emerged as promising modulators of reward-related brain circuits. This review synthesizes current evidence on the role of glucagon-like peptide-1 (GLP-1) and its receptor in modulating craving and substance-seeking behaviors. We highlight how GLP-1 receptors are expressed in addiction-relevant brain regions, including the ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC), where their activation influences dopaminergic, glutamatergic, and GABAergic neurotransmission. In addition, we explore how GLP-1 signaling affects reward processing through gut-brain vagal pathways, hormonal crosstalk, and neuroinflammatory mechanisms. Preclinical studies demonstrate that GLP-1RAs attenuate intake and relapse-like behavior across a range of substances, including alcohol, nicotine, and cocaine. Early-phase clinical trials support their safety and suggest potential efficacy in reducing craving. By integrating findings from molecular signaling, neurocircuitry, and behavioral models, this review provides a translational perspective on GLP-1RAs as an emerging treatment strategy in addiction medicine. We propose that targeting gut-brain metabolic signaling could provide a novel framework for understanding and treating SUDs.
Keywords: GLP-1 receptor agonist; addiction; craving; dopamine; gut–brain axis; mesolimbic pathway; neuroinflammation; reward circuitry; semaglutide; substance use disorder; synaptic plasticity; vagal signaling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
GLP-1 agonists: a game changer in pain treatment and addiction.Pain Manag. 2025 Jul 28:1-13. doi: 10.1080/17581869.2025.2536998. Online ahead of print. Pain Manag. 2025. PMID: 40726115 Review.
-
IUPHAR review - Glucagon-like peptide-1 (GLP-1) and substance use disorders: An emerging pharmacotherapeutic target.Pharmacol Res. 2024 Sep;207:107312. doi: 10.1016/j.phrs.2024.107312. Epub 2024 Jul 18. Pharmacol Res. 2024. PMID: 39032839 Free PMC article. Review.
-
The quantity, quality and findings of network meta-analyses evaluating the effectiveness of GLP-1 RAs for weight loss: a scoping review.Health Technol Assess. 2025 Jun 25:1-73. doi: 10.3310/SKHT8119. Online ahead of print. Health Technol Assess. 2025. PMID: 40580049 Free PMC article.
-
Glucagon-like peptide-1 receptor agonists and alcohol use disorders: An emerging unexpected beneficial effect.Diabetes Obes Metab. 2025 Aug;27(8):4083-4091. doi: 10.1111/dom.16453. Epub 2025 May 13. Diabetes Obes Metab. 2025. PMID: 40364515 Review.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Arlington, VA, USA: 2013. - DOI
-
- Mietlicki-Baase E.G., Ortinski P.I., Rupprecht L.E., Olivos D.R., Alhadeff A.L., Pierce R.C., Hayes M.R. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am. J. Physiol. Endocrinol. Metab. 2013;305:E1367–E1374. doi: 10.1152/ajpendo.00413.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

