Cost-Effectiveness of Ultra-Early Tranexamic Acid as Add-On to Standard Care After Subarachnoid Hemorrhage (ULTRA Trial)
- PMID: 40843772
- PMCID: PMC12371436
- DOI: 10.1111/ene.70208
Cost-Effectiveness of Ultra-Early Tranexamic Acid as Add-On to Standard Care After Subarachnoid Hemorrhage (ULTRA Trial)
Abstract
Objective: To evaluate the cost-effectiveness and cost-utility of adding ultra-early and short-term administration of tranexamic acid (TXA) to standard care in patients with subarachnoid hemorrhage (SAH).
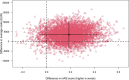
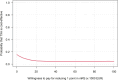
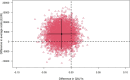
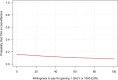
Materials and methods: An economic evaluation was performed alongside the ultra-early tranexamic acid after subarachnoid hemorrhage (ULTRA) trial. The main outcomes were the incremental cost-effectiveness ratio (ICER), expressed as costs per one-point increase in modified Rankin scale (mRS) score, and the incremental cost-utility ratio (ICUR), expressed as costs per quality-adjusted life-year (QALY). Cost-effectiveness acceptability curves (CEACs) were visualized with varying ICER cut-offs. Bootstrapping techniques and sensitivity analyses were performed to account for uncertainty.
Results: The ULTRA trial included 955 patients, with 480 assigned to the TXA group and 475 to the control group. The mean mRS score was 3.4 (95% CI: 3.2-3.5) in the TXA group and 3.2 (95% CI: 3.0-3.4) in the control group. The mean QALY was 0.26 (95% CI: 0.24-0.28) in the TXA group and 0.28 (95% CI: 0.26-0.30) in the control group. Mean costs were €62,180 (95% CI: 57,589-66,913) in the TXA group and €58,624 (95% CI: 53,693-63,955) in the control group. The probability of treatment with TXA being cost-effective ranged from 4% to 16% for mRS and from 8% to 16% for QALYs.
Conclusions: Ultra-early and short-term administration of TXA to patients with SAH is not cost-effective. Therefore, we recommend against using TXA for this patient group.
Trial registration: Netherlands Trial Register: NTR3272.
Clinicaltrials: gov identifier: NCT02684812.
Keywords: cost‐effectiveness; intracranial aneurysm; quality of life; subarachnoid hemorrhage; tranexamic acid.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Macdonald R. L. and Schweizer T. A., “Spontaneous Subarachnoid Haemorrhage,” Lancet 389, no. 10069 (2017): 655–666. - PubMed
-
- Nieuwkamp D. J., Setz L. E., Algra A., Linn F. H., de Rooij N. K., and Rinkel G. J., “Changes in Case Fatality of Aneurysmal Subarachnoid Haemorrhage Over Time, According to Age, Sex, and Region: A Meta‐Analysis,” Lancet Neurology 8, no. 7 (2009): 635–642. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

