Magnetic Resonance Cartography of Renal Tubule Volume Fraction During Diuretic Intervention
- PMID: 40843986
- PMCID: PMC12371858
- DOI: 10.1111/apha.70095
Magnetic Resonance Cartography of Renal Tubule Volume Fraction During Diuretic Intervention
Abstract
Aim: The renal tubular volume fraction (TVF) fluctuates under physiological conditions, and is altered in several renal diseases. Tools that enable noninvasive assessment of TVF are currently lacking. Magnetic Resonance (MR) TVF cartography is a novel approach for unraveling renal (patho-)physiology. Here, we employ MR-TVF cartography to monitor changes in response to the diuretic furosemide, and examine its role for the interpretation of renal oxygenation assessed by mapping the MRI relaxation time T2*. We hypothesize that furosemide increases TVF.
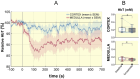
Methods: In anesthetized rats (n = 7) the MRI relaxation times T2, T2*, T2' and kidney size were obtained before/following an i.v. bolus of furosemide using a 9.4 Tesla MRI scanner. Spectral analysis of the T2 signal decay was performed to estimate the number of T2 components in renal tissue. TVF cartographies were calculated using voxel-wise bi-exponential fit of the T2 decay. Near Infrared Spectroscopy (NIRS, n = 9) was used to assess the total hemoglobin concentration (HbT) as a surrogate of renal blood volume.
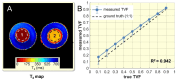
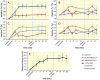
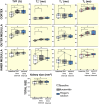
Results: Furosemide induced changes in renal MRI and NIRS parameters relative to baseline: TVFCORTEX = 31.1%, TVFOUTER_MEDULLA = 30.7%, T2_CORTEX = 13.0% and T2_OUTER_MEDULLA = 20.6%. HbTCORTEX was reduced by 2.7%. HbTMEDULLA declined by 8.6%. Kidney size showed a modest increase of 2.9%. T2*OUTER_MEDULLA and T2´OUTER_MEDULLA rose by 20.5% and 20.2%. T2*CORTEX and T2´CORTEX remained unchanged. T2* and TVF were strongly correlated in the outer medulla and moderately in the cortex.
Conclusion: MR-TVF cartography is highly relevant for elucidating mechanisms of renal (patho-)physiology, including the role of renal oxygenation assessed by MRI mapping of renal T2*.
Keywords: MRI; furosemide; kidney; tubule system; tubule volume fraction.
© 2025 The Author(s). Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Cao J., Zhu S., Ye M., et al., “Comparison of Renal Artery vs Renal Artery‐Vein Clamping During Partial Nephrectomy: A System Review and Meta‐Analysis,” Journal of Endourology 34, no. 4 (2020): 523–530. - PubMed
-
- Schoenberg S. O., Aumann S., Just A., et al., “Quantification of Renal Perfusion Abnormalities Using an Intravascular Contrast Agent (Part 2): Results in Animals and Humans With Renal Artery Stenosis,” Magnetic Resonance in Medicine 49, no. 2 (2003): 288–298. - PubMed
-
- Kellum J. A. and Prowle J. R., “Paradigms of Acute Kidney Injury in the Intensive Care Setting,” Nature Reviews. Nephrology 14, no. 4 (2018): 217–230. - PubMed
-
- Haase M., Bellomo R., Story D., et al., “Effect of Mean Arterial Pressure, Haemoglobin and Blood Transfusion During Cardiopulmonary Bypass on Post‐Operative Acute Kidney Injury,” Nephrology, Dialysis, Transplantation 27, no. 1 (2012): 153–160. - PubMed
-
- Li L., Fu H., and Liu Y., “The Fibrogenic Niche in Kidney Fibrosis: Components and Mechanisms,” Nature Reviews. Nephrology 18, no. 9 (2022): 545–557. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

