Time-Resolved Fluorescence Imaging and Correlative Cryo-Electron Tomography to Study Structural Changes of the HIV-1 Capsid
- PMID: 40844114
- PMCID: PMC12409903
- DOI: 10.1021/acsnano.5c06724
Time-Resolved Fluorescence Imaging and Correlative Cryo-Electron Tomography to Study Structural Changes of the HIV-1 Capsid
Abstract
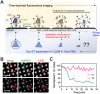
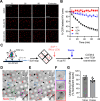
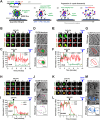
The conical HIV-1 capsid protects the internal viral genome and facilitates the infection of target cells. Highly potent antivirals, such as the clinically approved drug Lenacapavir (LEN), block HIV-1 replication by changing the capsid structure and modulating its function. However, structural studies of the HIV-1 capsid, its disassembly, or stabilization by antivirals have been challenging. Here, we developed a correlative light and cryo-electron microscopy (CLEM) workflow to characterize HIV-1 capsid morphology, starting from a small volume of viral particles harvested from cellular supernatants. We report two critical improvements in sample preparation, namely, (1) affinity capture and retention of fluorescent HIV-1 particles on cryo-EM grids to enable mapping virus/capsid location prior to sample vitrification and (2) streamlined alignment protocols to subsequently identify and correlate regions of interest in fluorescence and cryo-EM images. These improvements enable a reproducible CLEM workflow to accurately locate capsids for cryo-electron tomography (cryo-ET) studies. Using this approach, we resolved ultrastructures of HIV-1 capsids treated with LEN and the cellular metabolite inositol hexaphosphate (IP6), revealing distinct modes of capsid lattice stabilization. Finally, using our CLEM workflow, we demonstrate the feasibility of correlating time-resolved fluorescence imaging of capsid disassembly to end point cryo-ET structures. These advances will facilitate in vitro structural studies to define the mechanisms of HIV-1 capsid stabilization and disassembly. The CLEM workflow developed here can also be extended to studying structural changes in other viruses in response to diverse stimuli.
Keywords: CLEM; HIV-1 capsid uncoating; IP6; cryo-ET; lenacapavir.
Figures




Similar articles
-
Exploring the Structural Divergence of HIV and SRLV Lentiviral Capsids.J Am Chem Soc. 2025 Sep 10;147(36):32883-32895. doi: 10.1021/jacs.5c09436. Epub 2025 Aug 29. J Am Chem Soc. 2025. PMID: 40878534
-
Cryo-EM structure of the Seneca Valley virus A-particle and related structural states.J Virol. 2025 Aug 20:e0074425. doi: 10.1128/jvi.00744-25. Online ahead of print. J Virol. 2025. PMID: 40833065
-
Workflow for Fluorescence-Targeted Lamella Milling From Vitrified Cells With a Coincident Fluorescence, Electron, and Ion Beam Microscope.Bio Protoc. 2025 Jul 20;15(14):e5390. doi: 10.21769/BioProtoc.5390. eCollection 2025 Jul 20. Bio Protoc. 2025. PMID: 40741395 Free PMC article.
-
How HIV-1 Uses the Metabolite Inositol Hexakisphosphate to Build Its Capsid.Viruses. 2025 May 9;17(5):689. doi: 10.3390/v17050689. Viruses. 2025. PMID: 40431700 Free PMC article. Review.
-
Advancing Cryo-EM and Cryo-ET through Innovation in Sample Carriers: A Perspective.Anal Chem. 2025 Jun 17;97(23):11959-11967. doi: 10.1021/acs.analchem.5c01534. Epub 2025 Jun 6. Anal Chem. 2025. PMID: 40476672 Free PMC article. Review.
References
MeSH terms
Grants and funding
- S10 RR025080/RR/NCRR NIH HHS/United States
- U54 AI170855/AI/NIAID NIH HHS/United States
- P30 AG068635/AG/NIA NIH HHS/United States
- R24 GM154185/GM/NIGMS NIH HHS/United States
- R01 AI150998/AI/NIAID NIH HHS/United States
- S10 RR024564/RR/NCRR NIH HHS/United States
- U01 AI136680/AI/NIAID NIH HHS/United States
- U24 GM129539/GM/NIGMS NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
- T32 GM133351/GM/NIGMS NIH HHS/United States
- R24 GM145964/GM/NIGMS NIH HHS/United States
- R01 AI129862/AI/NIAID NIH HHS/United States
- F32 GM148049/GM/NIGMS NIH HHS/United States
- R21 AI174879/AI/NIAID NIH HHS/United States
- R01 AI184419/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

