Comparative Detection and Genetic Characterization of Feline Panleukopenia Virus in Bangladesh
- PMID: 40844648
- PMCID: PMC12372610
- DOI: 10.1002/vms3.70594
Comparative Detection and Genetic Characterization of Feline Panleukopenia Virus in Bangladesh
Abstract
Background: Feline panleukopenia virus (FPV) is a highly contagious and often fatal disease affecting domestic and wild felines. Accurate diagnosis and understanding of circulating strains are essential for effective control.
Objectives: This study aimed to evaluate the diagnostic accuracy of a rapid immunochromatographic (IC) antigen test compared to PCR for FPV detection in clinically suspected pet cats in Bangladesh. It also aimed to investigate the genetic and evolutionary characteristics of circulating FPV strains.
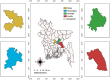
Methods: Faecal or rectal swab samples from suspected cats were tested using both IC strip tests and PCR. Sensitivity and specificity of the IC test were analysed using PCR as the reference. Partial sequencing of the VP2 gene was performed on four PCR-positive samples for phylogenetic and mutational analysis. Structural modelling of VP2 proteins was conducted to predict conformational changes.
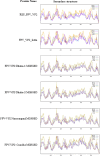
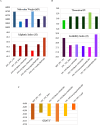
Results: The IC test detected FPV in 84% of cases, whereas PCR confirmed only 60%, indicating a 24% false-positive rate. PCR showed higher diagnostic reliability. FPV prevalence was 92% among unvaccinated cats. Phylogenetic analysis of VP2 sequences revealed close genetic similarity with Chinese and Portuguese strains, suggesting possible cross-border transmission. Mutations such as A756G, A896G, E299G and T236I were consistently observed. Structural modelling indicated minor conformational changes in VP2.
Conclusion and clinical significance: PCR offers superior accuracy over IC testing for FPV diagnosis. Mutational changes may impact antigenicity and diagnostic performance. Improved diagnostic accuracy, molecular surveillance and updated vaccination strategies are essential to control FPV outbreaks in feline populations.
Keywords: Feline panleukopenia virus; IC strip test; PCR; VP2 gene mutations; phylogenetic analysis.
© 2025 The Author(s). Veterinary Medicine and Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Abdelbaky, M. M. , El‐Khabaz K. A., and Hamed M. I.. 2024. “Diagnostic Performance of a Rapid in‐Clinic Test for the Detection of Feline Parvovirus.” Assiut Veterinary Medical Journal 70, no. 182: 253–262.
-
- Alessa, M. , Marzok M., Elnafarawy H., et al. 2026. “Prevalence of Feline panleukopenia (FPL) in Domestic Cats: A Systematic Meta‐Analysis.” Veterinary Integrative Sciences 24, no. 1: 1–17. 10.12982/VIS.2026.001. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

