Dual Immune Checkpoint Inhibition Plus Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Randomized Clinical Trial
- PMID: 40844778
- PMCID: PMC12374221
- DOI: 10.1001/jamanetworkopen.2025.27769
Dual Immune Checkpoint Inhibition Plus Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Randomized Clinical Trial
Abstract
Importance: Immune checkpoint inhibitors (ICIs) show efficacy in treatment of several solid tumors, but microsatellite-stable rectal cancer is largely resistant. Radiotherapy may enhance tumor immunogenicity and thus may make the combination of radiotherapy and ICIs a promising strategy to treat rectal cancer. While anti-programmed cell death protein 1 antibodies in neoadjuvant regimens have been linked to higher complete response rates, the added benefit of including a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor remains unclear.
Objective: To evaluate the safety and feasibility of combining ipilimumab and nivolumab with neoadjuvant chemoradiotherapy (CRT) for rectal cancer.
Design, setting, and participants: The CHINOREC trial was a prospective, randomized, open-label, multicenter phase 2 clinical trial conducted from June 2, 2020, to March 15, 2024, across multiple academic and tertiary medical centers in Austria. Analysis was based on intention to treat.
Intervention: Neoadjuvant CRT consisted of 50 Gy in 2-Gy fractions with concurrent capecitabine (1650 mg/m2/d). The experimental arm received additional intravenous ipilimumab (1 mg/kg on day 7) and nivolumab (3 mg/kg every 2 weeks starting on day 14) (CRT plus ipilimumab and nivolumab group). Surgical resection was performed 10 to 12 weeks after CRT.
Main outcome and measures: The primary outcome was the safety and feasibility of combining CRT with sequential ipilimumab and nivolumab, assessed by surgical complications and reoperation rates. Secondary outcomes included clinical and pathological response rates.
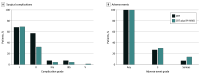
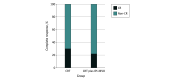
Results: Of the 145 patients assessed, 80 were randomized to receive either CRT alone (CRT group) (n = 30) or to the CRT plus ipilimumab and nivolumab group (n = 50) (49 male [61%]; median age, 60 [range, 36-83] years). Differences in surgical complication rates were not statistically significant between the CRT and CRT plus ipilimumab and nivolumab groups (any grade, 20 of 26 patients [77%] vs 33 of 43 [77%]; P > .99), as were reoperation rates (2 of 26 [8%] vs 3 of 43 [7%]; P > .99). Major pathological response (10 of 26 [38%] vs 16 of 43 [37%]; P > .99) and complete response (9 of 30 [30%] vs 11 of 50 [22%]; P = .44) rates were overall high in both groups.
Conclusions and relevance: In this randomized clinical trial of patients with rectal cancer, integrating ipilimumab and nivolumab into neoadjuvant CRT was safe and feasible, with no increase in surgical complications. Although complete response rates did not significantly improve, the dual ICI regimen demonstrated promising clinical activity. These findings support further translational research to optimize timing, dosing, and fractionation of radiotherapy and ICI therapy and to guide patient selection.
Trial registration: ClinicalTrials.gov Identifier: NCT04124601.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

