Ischemic duration determines extent of cardiac remodeling, and both early and delayed reperfusion prevent fatal cardiac rupture: Model comparison
- PMID: 40844997
- PMCID: PMC12373173
- DOI: 10.1371/journal.pone.0328001
Ischemic duration determines extent of cardiac remodeling, and both early and delayed reperfusion prevent fatal cardiac rupture: Model comparison
Abstract
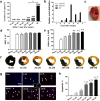
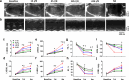
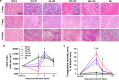
High incidence of cardiac rupture in murine myocardial infarction (MI) model leads to a substantial loss before the study end-point. Selecting animal models with varying degrees of injury for different research purposes is crucial for cardiovascular research. Male C57 mice were subjected to ischemia/reperfusion (I/R) or permanent occlusion (MI) injury. The incidence of cardiac rupture, degree of myocardial injury, inflammatory responses, left ventricular (LV) remodeling and infarct myocardium healing were examined. Compared to MI mice, early reperfusion (1, 2 and 4h I/R) completely prevented cardiac rupture, while delayed reperfusion (12h and 24h I/R) significantly reduced incidence of cardiac rupture to 5.7% and 8.6%, respectively. In the acute phase, prolonged ischemia increased infarct size, myocyte apoptosis, and both systemic and regional inflammatory responses. These changes correspond to enhanced MMP-9 activity and a weakening of the tensile strength of the infarcted myocardium. Following ischemic insult, early reperfusion was associated with less extent of myocardial injury, inflammatory response and adverse cardiac remodeling, whereas, delayed reperfusion and MI groups exhibited severe myocardial damage and remodeling. Furthermore, both early and delayed reperfusion were associated with increased infiltration of type 2 macrophages and proliferation of endothelial cells during the early healing phase, thereby facilitating healing of the infarct myocardium. Delayed reperfusion resulted in a comparable and substantial degree of cardiac remodeling but with a lower risk of cardiac rupture in comparison with MI model. This feature makes it a feasible model for cardiac ischemia research.
Copyright: © 2025 Zhao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Macro- and microinjury define the heart failure progression after permanent coronary ligation or ischemia-reperfusion in young healthy mice.Am J Physiol Heart Circ Physiol. 2025 Aug 1;329(2):H521-H533. doi: 10.1152/ajpheart.00267.2025. Epub 2025 Jul 16. Am J Physiol Heart Circ Physiol. 2025. PMID: 40668645
-
HuR inhibition reduces post-ischemic cardiac remodeling by dampening myocyte-dependent inflammatory gene expression and the innate immune response.FASEB J. 2025 Mar 31;39(6):e70433. doi: 10.1096/fj.202400532RRR. FASEB J. 2025. PMID: 40085190 Free PMC article.
-
CD11b Blockade Ameliorates Myocardial Ischemia/Reperfusion Injury by Reducing Neutrophil and Monocyte Infiltration.J Am Heart Assoc. 2025 Jul;14(13):e038142. doi: 10.1161/JAHA.124.038142. Epub 2025 Jun 27. J Am Heart Assoc. 2025. PMID: 40576028
-
Remote Ischemic Perconditioning to Reduce Reperfusion Injury During Acute ST-Segment-Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis.J Am Heart Assoc. 2017 May 17;6(5):e005522. doi: 10.1161/JAHA.117.005522. J Am Heart Assoc. 2017. PMID: 28515120 Free PMC article.
-
Protective effect of sevoflurane on myocardial ischemia-reperfusion injury: a systematic review and meta-analysis.Int J Surg. 2024 Nov 1;110(11):7311-7330. doi: 10.1097/JS9.0000000000001975. Int J Surg. 2024. PMID: 39093878 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

