Cardiac function and extracellular matrix morphology are altered by chronic high fat diet in Drosophila larvae
- PMID: 40845064
- PMCID: PMC12373202
- DOI: 10.1371/journal.pone.0330487
Cardiac function and extracellular matrix morphology are altered by chronic high fat diet in Drosophila larvae
Abstract
Cardiovascular disease is characterized by aberrant and excessive extracellular matrix (ECM) remodelling, termed fibrosis. Fibrotic remodelling is typically triggered by inflammation, which occurs systemically in obesity. Despite the contribution of fibrosis to adverse clinical outcomes and disease progression, there are no available treatments for this condition. Developing therapeutics for chronic conditions requires an understanding of in vivo ECM regulation, and how the ECM responds to a systemic challenge. We have therefore developed a Drosophila model for obesity via chronic high fat diet feeding of larvae and evaluated the response of the cardiac ECM to this metabolic challenge. We found that this model displays a striking reorganisation of the cardiac ECM, with fibres oriented anterior to posterior, rather than in a complex network, suggesting tension modulation is altered. We also observe corresponding deficits in heart function, with high fat diet treatments resulting in an inability to contract the heart effectively at systole. Our study reveals that different genotypes tolerate different levels of dietary fat, and that some genotypes may require a different dietary supplementation regime to generate a cardiac phenotype. In summary, the Drosophila model for chronic high fat diet recapitulates many of the defects observed in human cardiovascular disease, allowing further evaluation of genetic and environmental influences on cardiac structure and physiology in disease states.
Copyright: © 2025 Andrews et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
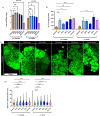

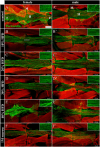
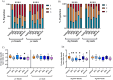

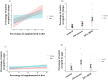
References
-
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

