Association of Enlarged Perivascular Spaces With Early Serum and Neuroimaging Biomarkers of Alzheimer Disease Pathology
- PMID: 40845262
- PMCID: PMC12377920
- DOI: 10.1212/WNL.0000000000213836
Association of Enlarged Perivascular Spaces With Early Serum and Neuroimaging Biomarkers of Alzheimer Disease Pathology
Erratum in
-
Association of Enlarged Perivascular Spaces With Early Serum and Neuroimaging Biomarkers of Alzheimer Disease Pathology.Neurology. 2025 Nov 11;105(9):e214339. doi: 10.1212/WNL.0000000000214339. Epub 2025 Oct 15. Neurology. 2025. PMID: 41092251 No abstract available.
Abstract
Background and objectives: Enlarged perivascular spaces (EPVS), recognized as a key feature of cerebral small vessel disease (CSVD), have emerged as a promising biomarker for vascular contribution to Alzheimer disease (AD) and other neurodegenerative diseases. Although previous studies have linked EPVS to cerebrovascular dysfunction, their relationship with AD pathology and cognitive decline remains underexplored, particularly in multiethnic cohorts. This study investigates the associations between basal ganglia EPVS burden, blood-based biomarkers (BBM), and cognitive outcomes in a Southeast Asian cohort.
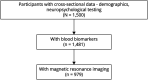
Methods: This cross-sectional study drew from the Biomarkers and Cognition Study, Singapore, comprising participants recruited from the community, at Dementia Research Centre (Singapore) from 2022 to 2024. Participants underwent comprehensive neuropsychologic assessments and were classified into cognitively normal, subjective cognitive decline, and mild cognitive impairment (MCI) groups according to established diagnostic criteria. BBM including amyloid β oligomers, amyloid β42 (Aβ42) and β40 (Aβ40), phosphorylated tau 181 (p-tau181), neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP) were quantified. MRI Markers of CSVD (EPVS, white matter hyperintensities [WMH], lacunes, and microbleeds) were visually rated using validated scales. Associations between EPVS, biomarkers, and cognitive outcomes were analyzed using correlation or multivariable regression models adjusting for age, sex, education, cognitive diagnosis, and APOE ε4 carrier status.
Results: A total of 979 participants were included (mean age: 58.2 ± 10.7 years; mean education: 14.9 ± 3.5 years; 60.7% female). Elevated EPVS burden was positively correlated with higher GFAP (ρ = 0.166, 95% CI 0.104 to 0.228, p < 0.01), NfL (ρ = 0.169, 95% CI 0.112 to 0.242, p < 0.01), and p-tau181 (ρ = 0.087, 95% CI 0.022 to 0.152, p < 0.01) and inversely with the Aβ42/40 ratio (ρ = -0.077, 95% CI -0.144 to 0.006, p < 0.05)-suggesting links to neuroinflammation and amyloid pathology. Among CSVD markers, EPVS exhibited the strongest association with Aβ pathology in participants with MCI (odds ratio [OR] 1.877, 95% CI 1.045 to 3.370, p = 0.035). In addition, higher EPVS burden was linked to poorer visuospatial skills and executive function (Block Design Test, OR 0.182, 95% CI 0.037 to 0.890, p = 0.035).
Discussion: These findings suggest EPVS burden to be a potential marker of both CSVD and AD-related BBM pathology. The results support the potential of incorporating EPVS assessments into routine MRI evaluations to enhance early detection and risk stratification in AD. Longitudinal studies are needed to confirm the prognostic value of EPVS and to clarify mechanisms linking vascular dysfunction to amyloid and tau pathology.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures

References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
-
- World Health Organisation. Dementia. Accessed February 10, 2025. who.int/news-room/fact-sheets/detail/dementia
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
