Controlled Molecular Orders in Layered Multiple Porphyrins
- PMID: 40846649
- PMCID: PMC12412108
- DOI: 10.1021/jacs.5c07484
Controlled Molecular Orders in Layered Multiple Porphyrins
Abstract
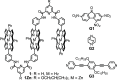
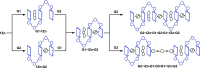
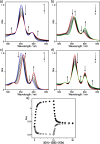
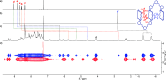
Nature precisely regulates multicomponent assemblies with the assistance of cooperativity. However, establishing such high precision in multicomponent assemblies of artificial supramolecular structures remains challenging. Here, we successfully position multiple distinct guest molecules within two equivalent binding cavities of a zinc-metalated trisporphyrin host by combining two distinct negative cooperative interactions, including donor-acceptor π-stacking and metal-ligand coordination. Comprehensive characterization using UV-vis absorption spectroscopy and diffusion-ordered NMR spectroscopy confirmed the exclusive formation of a ternary supramolecular complex. X-ray crystallographic analysis further revealed that the introduction of an additional bridging ligand effectively linked the two ternary complexes to produce an unprecedented septenary supramolecular assembly with alternating guest sequences. In contrast to conventional methods, which require structural differentiation or positive cooperativity, our strategy relies exclusively on negative cooperativity to achieve highly precise molecular ordering. This study presents a novel approach toward constructing sophisticated multicomponent molecular assemblies, emphasizing the significant but underutilized role of negative cooperativity in achieving molecular precision in artificial supramolecular chemistry.
Figures



References
-
- Margetic D.. Host-guest Studies of Bis-porphyrins. Curr. Org. Chem. 2012;16:829–851. doi: 10.2174/138527212800194809. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

