Prolonging lung cancer response to EGFR inhibition by targeting the selective advantage of resistant cells
- PMID: 40846697
- PMCID: PMC12373916
- DOI: 10.1038/s41467-025-61788-w
Prolonging lung cancer response to EGFR inhibition by targeting the selective advantage of resistant cells
Abstract
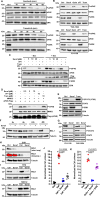
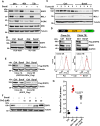
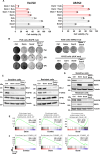
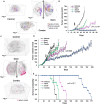
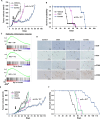
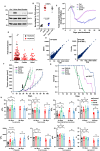
Non-small cell lung cancers (NSCLCs) treated with tyrosine kinase inhibitors (TKIs) of the epidermal growth factor receptor (EGFR) almost invariably relapse in the long term, due to the emergence of subpopulations of resistant cells. Through a DNA barcoding approach, we show that the clinically approved drug sorafenib specifically abolishes the selective advantage of EGFR-TKI-resistant cells, while preserving the response of EGFR-TKI-sensitive cells. Sorafenib is active against multiple mechanisms of resistance/tolerance to EGFR-TKIs and its effects depend on early inhibition of MAPK-interacting kinase (MKNK) activity and signal transducer and activator of transcription 3 (STAT3) phosphorylation, and later down-regulation of MCL1 and EGFR. Using different xenograft and allograft models, we show that the sorafenib-EGFR-TKI combination can delay tumor growth and promote the recruitment of inflammatory cells. Together, our findings indicate that sorafenib can prolong the response to EGFR-TKIs by targeting NSCLC capacity to adapt to treatment through the emergence of resistant cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Z.K. reports financial support from DeuterOncology NV outside the submitted work. B.C.C. reports stock ownership with TheraCanVac Inc, Gencurix Inc, Bridgebio therapeutics, KANAPH Therapeutic Inc, Cyrus Therapeutics, Interpark Bio Convergence Corp and J INTS BIO; reports participating in an advisory role for KANAPH Therapeutic Inc, Brigebio Therapeutics, Cyrus Therapeutics, Guardant Health and Oscotec; has received consulting fees from Novartis, Abion, BeiGene, AstraZeneca, Boehringer-Ingelheim, Roche, Bristol-Myers Squibb, ONO, Yuhan, Pfizer, Eli Lilly, Janssen, Takeda, MSD, Janssen, Medpacto, and Blueprint medicines; has received grants or funds from Novartis, Bayer, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, ONO, Dizal Pharma, MSD, Abbvie, Medpacto, GI Innovation, Eli Lilly, Blueprint medicines, and Interpark Bio Convergence Corp; has received royalties from Campions Oncology, Crown Bioscience and Imagen; and is the founder of DAAN Biotherapeutics. A.B.C. has received honorarium for advisory positions, board memberships, lectures, or non-financial support from the following sources: Astra-Zeneca, Roche, MSD, Pfizer, Novartis, Takeda, Janssen, AbbVie, and Amgen. L.G. is inventor of a patent on DNA barcoding issued to Inserm and University of Rouen (WO2017068120A1). S. A. reports personal fees from MSD Italia and a patent (Italian patent application No. 102022000007535) outside the submitted work. A.B. reports receipt of grants/research supports from Neophore, AstraZeneca, Boehringer Ingelheim, and honoraria/consultation fees from Guardant Health. A.B. is stock shareholder of Neophore and Kither Biotech. A.B. is advisory boards member for Neophore. The remaining authors declare no competing interests.
Figures


References
-
- Peters, S., Mok, T., Passaro, A. & Janne, P. A. The promising evolution of targeted therapeutic strategies in cancer. Cancer Discov.11, 810–814 (2021). - PubMed
-
- Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med.378, 113–125 (2018). - PubMed
-
- Ramalingam, S. S. et al. Osimertinib as first-line treatment of egfr mutation-positive advanced non-small-cell lung cancer. J. Clin. Oncol.36, 841–849 (2018). - PubMed
-
- Passaro, A., Janne, P. A., Mok, T. & Peters, S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat. Cancer2, 377–391 (2021). - PubMed
-
- Hanna, N. H. et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J. Clin. Oncol.39, 1040–1091 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

