Episodic transport of protein aggregates achieves a positive size selectivity in aggresome formation
- PMID: 40846843
- PMCID: PMC12373839
- DOI: 10.1038/s41467-025-62751-5
Episodic transport of protein aggregates achieves a positive size selectivity in aggresome formation
Abstract
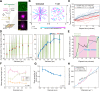
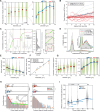
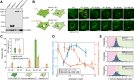
Eukaryotic cells direct toxic misfolded proteins to various quality control pathways based on their chemical properties and aggregation status. Aggregated proteins are targeted to selective autophagy or specifically sequestered into the "aggresome", a perinuclear inclusion at the microtubule-organizing center (MTOC). However, the mechanism for selective aggresome recruitment remains unclear. To investigate this process, here we reconstitute MTOC-directed aggregate transport in Xenopus laevis egg extract using AgDD, a chemically inducible aggregation system. High-resolution single-particle tracking reveals that dynein-mediated aggregate transport is highly episodic, with average velocity positively correlating with aggregate size. Mechanistic modeling suggests that recurrent formation of the dynein transport complex biases larger aggregates towards active transport, compensating for the slowdown due to viscosity. Both episodic transport and positive size selectivity are conferred by aggresome-specific dynein adapters. Coupling an aggresome adapter to polystyrene beads recapitulates positive size selectivity in transport, while recruiting conventional dynein adapters to protein aggregates perturbs aggresome formation and reverses the size selectivity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors claim no competing interests.
Figures



Update of
-
Episodic Transport of Protein Aggregates Achieves a Positive Size Selectivity in Aggresome Formation.bioRxiv [Preprint]. 2024 Aug 7:2024.08.06.606767. doi: 10.1101/2024.08.06.606767. bioRxiv. 2024. Update in: Nat Commun. 2025 Aug 22;16(1):7852. doi: 10.1038/s41467-025-62751-5. PMID: 39211171 Free PMC article. Updated. Preprint.
References
-
- Johnston, H. E. & Samant, R. S. Alternative systems for misfolded protein clearance: life beyond the proteasome. FEBS J. 10.1111/febs.15617 (2020). - PubMed
-
- Olanow, C. W., Perl, D. P., DeMartino, G. N. & McNaught, K. S. P. Lewy-body formation is an aggresome-related process: a hypothesis. Lancet Neurol.3, 496–503 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

