Harnessing artificial intelligence to identify Bufalin as a molecular glue degrader of estrogen receptor alpha
- PMID: 40846852
- PMCID: PMC12373995
- DOI: 10.1038/s41467-025-62288-7
Harnessing artificial intelligence to identify Bufalin as a molecular glue degrader of estrogen receptor alpha
Abstract
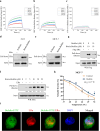
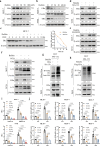
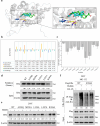
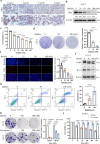
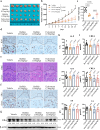
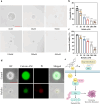
Target identification in natural products plays a critical role in the development of innovative drugs. Bufalin, a compound derived from traditional medicines, has shown promising anti-cancer activity; however, its precise molecular mechanism of action remains unclear. Here, we employ artificial intelligence, molecular docking, and molecular dynamics simulations to elucidate the molecular mechanism of Bufalin. Using an integrated multi-predictive strategy, we identify CYP17A1, ESR1, mTOR, AR, and PRKCD as the potential targets of Bufalin. Subsequent validation via surface plasmon resonance, biotin pulldown, and thermal shift assays confirms Bufalin's direct binding to ESR1, which encodes estrogen receptor alpha (ERα). Molecular docking analyses pinpoint Bufalin's selective interaction with Arg394 on ERα. Molecular dynamic simulations further show that Bufalin acts as a molecular glue, enhancing the interaction between ERα and the E3 ligase STUB1, thereby promoting proteasomal degradation of ERα. Given the therapeutic potential of ERα degradation in overcoming endocrine resistance, we investigate the inhibitory effect of Bufalin on endocrine-resistant models and prove Bufalin reverses Tamoxifen resistance in vitro, in vivo, and in patient-derived breast cancer organoids from tamoxifen-relapsed cases. Collectively, our findings indicate that Bufalin functions as a molecular glue to degrade ERα, offering a potential therapeutic strategy for reversing Tamoxifen resistance.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Chen, J. et al. Bufalin targets the SRC-3/MIF pathway in chemoresistant cells to regulate M2 macrophage polarization in colorectal cancer. Cancer Lett.513, 63–74 (2021). - PubMed
-
- Soumoy, L., Ghanem, G. E., Saussez, S. & Journe, F. Bufalin for an innovative therapeutic approach against cancer. Pharm. Res.184, 106442 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

