Dissolving microneedles enabled delivery of Oxaliplatin- sodium butyrate loaded outer membrane vesicles against rectal cancer
- PMID: 40847351
- PMCID: PMC12372368
- DOI: 10.1186/s12967-025-06921-5
Dissolving microneedles enabled delivery of Oxaliplatin- sodium butyrate loaded outer membrane vesicles against rectal cancer
Abstract
Background: Oxaliplatin (OXA) is a commonly used drug for the treatment of rectal cancer (RC). However, traditional administration methods are plagued by low efficiency, high systemic side effects, and poor patient tolerance. Therefore, there is an urgent need to develop a targeted drug delivery system to enhance efficacy and reduce toxicity.
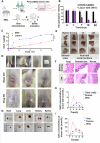
Methods: A strain of Clostridium butyricum with anti-cancer properties was screened, and outer membrane vesicles (OMVs) rich in sodium butyrate (NaB) were prepared. These OMVs (NaB-OMVs, NOMVs) were used to address the challenge of combined administration caused by differences in the administration methods and physicochemical properties of OXA and NaB. OXA was encapsulated into NOMVs via sonication to obtain OXA-loaded NaB-OMVs (OXA@NOMVs, ONOMVs). To improve stability and enable targeted delivery, a dissolving microneedle (MNs) system (ONOMVs@MNs) was developed using PVP K90 and sodium hyaluronate via the mold method. The system was characterized for morphology, size, zeta potential, mechanical strength, and rectal mucosa permeability. Additionally, targeted therapy advantages were evaluated using methods including anal microneedle administration in mice and fluorescence labeling of vesicles.
Results: Characterization showed that ONOMVs exhibit a saucer-like morphology with a diameter of 100 nm and a zeta potential of 20 mV. The ONOMVs@MNs were conically shaped and possessed sufficient mechanical strength to penetrate the anal mucosa. In rectal mucosa permeability experiments, the 2-hour permeability of the MNs group was 1.78 times higher than that of the liquid (liquor) group. Anal microneedle administration in mice and fluorescence labeling confirmed the targeted therapy advantages of NOMVs@MNs.
Conclusions: ONOMVs@MNs is an efficient local drug delivery platform that combines NaB and OXA, providing a potential therapeutic approach for RC.
Keywords: Microneedles; Outer membrane vesicles; Oxaliplatin; Rectal cancer; Sodium butyrate.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The clinical protocols involving the patients and the informed consent form were approved by the Ethics Committee of Huzhou Central Hospital (No.202312005-01). The animal experiments were approved by the Experimental Animal Management and Ethics Committee of Huzhou Central Hospital (No.202401001). Consent for publication: All authors read and approved the final manuscript. Competing interests: The authors declare that no potential conflicts of interest exist.
Figures






References
-
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–59. - PubMed
-
- Shuwen H, Xi Y, Yuefen P, Jiamin X, Quan Q, Haihong L, Yizhen J, Wei W. Effects of postoperative adjuvant chemotherapy and palliative chemotherapy on the gut Microbiome in colorectal cancer. Microb Pathog. 2020;149:104343. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

