Efficient induction of tetraploids via adventitious bud regeneration and subsequent phenotypic variation in Acacia melanoxylon
- PMID: 40847368
- PMCID: PMC12372237
- DOI: 10.1186/s13007-025-01426-0
Efficient induction of tetraploids via adventitious bud regeneration and subsequent phenotypic variation in Acacia melanoxylon
Abstract
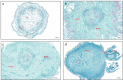
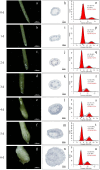
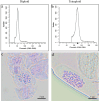
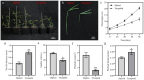
BACKGROUND ACACIA MELANOXYLON: is an important species for establishing pulpwood plantations due to its high application value in engineered wood products. However, the lack of a well-established in vitro regeneration system has severely constrained its industrial-scale propagation and the induction of tetraploids. RESULTS: In this study, using the superior A. melanoxylon clone SR3, an in vitro regeneration system using a bud-bearing stem segment was established. A DKW medium supplemented with 0.5 mg/L 6-BA, 0.1 mg/L IAA, and 0.2 mg/L NAA was determined as the optimal differentiation medium. Adding 0.5 mg/L IBA and 0.25 mg/L NAA to the 1/2 MS medium produced a higher rooting percentage and root number. To determine the optimal timing for tetraploid induction in A. melanoxylon, morphological, cytological, and flow cytometric analyses were conducted on the swollen tissue at the base of the bud-bearing stem segment. On the 5th day of preculture, white callus tissue was observed, characterized by vigorous cell division and the highest G2/M-phase cell content in the adventitious bud primordia. After colchicine treatment, the tetraploid induction efficiency on the 5th day of preculture was significantly higher compared to the 4th or 6th day. The highest induction rate of 12.26 ± 0.80% was achieved with 100 mg/L colchicine for 72 h on the 5th day of preculture. Furthermore, tetraploid A. melanoxylon exhibited morphological traits such as reduced plant height, leaf number, and stomatal density. CONCLUSIONS: This study establishes a stable and effective method for in vitro tetraploid induction in A. melanoxylon, providing theoretical and technical support for polyploid breeding and laying the groundwork for subsequent triploid development.
Keywords: Acacia tree; Adventitious bud; Bud-bearing stem segment; Developmental stage observation; Morphological variation; Tetraploid induction.
© 2025. The Author(s).
Conflict of interest statement
Ethics. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Efficient callus induction and regeneration of tea plant (Camellia sinensis).Sci Rep. 2025 Jul 23;15(1):26848. doi: 10.1038/s41598-025-12271-5. Sci Rep. 2025. PMID: 40702144 Free PMC article.
-
Highly Efficient Regeneration of Bombax ceiba via De Novo Organogenesis from Hypocotyl and Bud Explants.Plants (Basel). 2025 Jul 2;14(13):2033. doi: 10.3390/plants14132033. Plants (Basel). 2025. PMID: 40648042 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Celesti-Grapow L, Bassi L, Brundu G, Camarda I, Carli E, D’Auria G, et al. Plant invasions on small mediterranean islands: an overview. Plant Biosyst. 2016;150(5):1119–33.
-
- Chemetova C, Ribeiro H, Fabiao A, Gominho J. Towards sustainable valorisation of Acacia melanoxylon biomass: characterization of mature and juvenile plant tissues. Environ Res. 2020;191:110090. - PubMed
-
- Bradbury GJ, Potts BM, Beadle CL. Genetic and environmental variation in wood properties of Acacia melanoxylon. Ann for Sci. 2011;68(8):1363–73.
-
- Machado JS, Louzada JL, Santos AJ, Nunes L, Anjos O, Rodrigues J, et al. Variation of wood density and mechanical properties of Blackwood (Acacia melanoxylon R. Br). Mater Des. 2014;56:975–80.
-
- Bradbury GJ, Beadle CL, Potts BM. Genetic control in the survival, growth and form of Acacia melanoxylon. New for. 2010;39(2):139–56.
LinkOut - more resources
Full Text Sources

