Synthetic Peptides Suppress Nervous Necrosis Virus Absorption and Improve Survival Rates in European Sea Bass
- PMID: 40848122
- PMCID: PMC12374861
- DOI: 10.1007/s10126-025-10507-z
Synthetic Peptides Suppress Nervous Necrosis Virus Absorption and Improve Survival Rates in European Sea Bass
Abstract
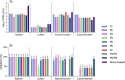
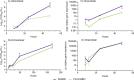
With few preventive strategies available against nodavirus (NNV) in aquaculture, therapeutic applications remain underexplored. This study aimed to peptide-based treatments disrupting critical stages of its viral life cycle. Thus, we designed and synthesized seven low-molecular-weight peptides (P1-P7) based on predicted binding regions of the capsid protein from the red-spotted grouper nervous necrosis virus (RGNNV) genotype to mimic viral capsid regions. Although in silico predictions suggested limited direct antiviral activity, in vitro assays using the E-11 cell line and in vivo trials in RGNNV-infected European sea bass (Dicentrarchus labrax) juveniles yielded promising results. The peptides, particularly when co-administered individually or as P3 + P4 and P5 + P6 combinations with the virus, disrupted RGNNV attachment in vitro. Moreover, they exhibited cross-reactivity against the striped jack nervous necrosis virus (SJNNV) genotype and both RGNNV/SJNNV and SJNNV/RGNNV reassortants. Treatment of RGNNV-infected sea bass significantly increased the relative percent survival, ranging from 81.3% for P4 to 62.5% for P3 and P3 + P4, while reducing viral load within 48 h post-treatment without altering systemic antiviral immune responses, tested through the transcriptional levels of mx gene in the head-kidney. Notably, peptide P4 partially inhibited viral replication in vitro at the same time-point when cells were pre-treated for 24 h, likely through modulation of host immune responses. These findings highlight the potential of targeted peptide-based therapies as a promising antiviral therapeutic strategy against NNV infections.
Keywords: Adsorption; Betanodavirus; European sea bass; Replication; Synthetic peptides; Viral cycle.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Genomic Differences Between Nervous Necrosis Virus (NNV) Reassortants Isolated From Wild and Farmed Fish: Implications for Viral Fitness, Temperature Adaptation and Virulence.Transbound Emerg Dis. 2025 Jun 18;2025:8896753. doi: 10.1155/tbed/8896753. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40567258 Free PMC article.
-
Influence of Viral Re-Infection on Head Kidney Transcriptome of Nervous Necrosis Virus-Resistant and -Susceptible European Sea Bass (Dicentrarchus labrax, L.).Viruses. 2025 Feb 7;17(2):230. doi: 10.3390/v17020230. Viruses. 2025. PMID: 40006985 Free PMC article.
-
Lack of in vivo cross-protection of two different betanodavirus species RGNNV and SJNNV in European sea bass Dicentrachus labrax.Fish Shellfish Immunol. 2019 Feb;85:85-89. doi: 10.1016/j.fsi.2017.10.033. Epub 2017 Oct 19. Fish Shellfish Immunol. 2019. PMID: 29056488
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Bajaj S, Banerjee M (2016) In vitro assembly of polymorphic virus-like particles from the capsid protein of a nodavirus. Virology 496:106–115 - PubMed
-
- Barsøe S, Toffan A, Pascoli F, Stratmann A, Pretto T, Marsella A, Er-Rafik M, Vendramin N, Olesen NJ, Sepúlveda D, Lorenzen N (2021) Long-term protection and serologic response of European sea bass vaccinated with a betanodavirus virus-like particle produced in Pichia pastoris. Vaccines 9:447 - PMC - PubMed
-
- Castric J, Thiery R, Jeffroy J, de Kinkelin P, Raymond JC (2001) Sea bream Sparus aurata, an asymptomatic contagious fish host for nodavirus. Dis Aquat Organ 47:33–38 - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- PID2022-139492NB-I00/Ministerio de Ciencia, Innovación y Universidades
- PREP2022-000442/Ministerio de Ciencia, Innovación y Universidades
- PID2022-139492NB-I00/European Regional Development Fund
- PREP2022-000442/European Social Fund Plus
- IJC2020-042733-I/Ministerio de Ciencia, Innovación y Universidades,Spain
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
