Nanoengineered hydrogels disrupt tumor antioxidant defense via photothermal-chemodynamic synergy and oxidative stress boosts
- PMID: 40849478
- PMCID: PMC12374360
- DOI: 10.1186/s12951-025-03626-1
Nanoengineered hydrogels disrupt tumor antioxidant defense via photothermal-chemodynamic synergy and oxidative stress boosts
Abstract
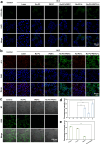
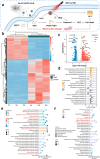
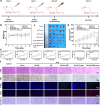
By integrating photothermal and chemodynamic properties, Ru-based nanomaterials have emerged as promising agents for tumor therapy. However, their clinical translation is hindered by high systemic toxicity, suboptimal therapeutic efficacy, and compromised chemodynamic performance caused by tumor antioxidant defense mechanisms. A multifunctional therapeutic platform (Ru-PC-PEITC-ALG) was developed through the coordination-driven self-assembly of ruthenium ions with procyanidins (PCs) to form Ru-PC nanoparticles, followed by coencapsulation with phenethyl isothiocyanate (PEITC) in a sodium alginate hydrogel. The Ru-PC complex demonstrated exceptional photothermal conversion efficiency, enabling rapid intratumoral temperature elevation under 808 nm laser irradiation to achieve localized thermal ablation. Simultaneously, Ru-PC exhibited tumor microenvironment-responsive catalytic activity, catalyzing the conversion of hydrogen peroxide (H2O2) into highly toxic hydroxyl radicals (·OH) via Fenton-like reactions. This ROS generation was substantially amplified through synergistic photothermal acceleration of reaction kinetics and PEITC-mediated glutathione (GSH) depletion, which effectively disabled the antioxidant defense system. Systematic evaluations, including in vitro cytotoxicity assays, transcriptomic sequencing, and murine xenograft models, confirmed the platform's superior tumor suppression ability and favorable biosafety profile. Mechanistic studies revealed that combination therapy induced mitochondrial dysfunction and activated the apoptosis/ferroptosis pathways. This work presents a "precision disruption" strategy against tumor antioxidant armor, advancing the rational design of metal‒polyphenol-coordinated nanomaterials for enhanced oncotherapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal studies have been approved by the Ethics Committee of Anhui Medical University (No. LLSC20220731) and all handling of mice was performed in accordance with the institutional regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Copper-doped PDA nanoparticles with self-enhanced ROS generation for boosting photothermal/chemodynamic combination therapy.Biomater Sci. 2025 Jul 8;13(14):3903-3914. doi: 10.1039/d5bm00418g. Biomater Sci. 2025. PMID: 40478243
-
H2O2 self-supplying cascade catalytic nanoreactors amplify oxidative stress for augmented cuproptosis-driven multimodal synergistic therapy of breast cancer.Colloids Surf B Biointerfaces. 2025 Oct;254:114802. doi: 10.1016/j.colsurfb.2025.114802. Epub 2025 May 16. Colloids Surf B Biointerfaces. 2025. PMID: 40383022
-
Redox homeostasis disruptors enhanced cuproptosis effect for synergistic photothermal/chemodynamic therapy.J Colloid Interface Sci. 2025 Jan 15;678(Pt A):1060-1074. doi: 10.1016/j.jcis.2024.08.234. Epub 2024 Aug 31. J Colloid Interface Sci. 2025. PMID: 39236435
-
Innovative approaches for cancer treatment: graphene quantum dots for photodynamic and photothermal therapies.J Mater Chem B. 2024 May 8;12(18):4307-4334. doi: 10.1039/d4tb00255e. J Mater Chem B. 2024. PMID: 38595268 Review.
-
Composite Nanomaterials of Conjugated Polymers and Upconversion Nanoparticles for NIR-Triggered Photodynamic/Photothermal Synergistic Cancer Therapy.ACS Appl Mater Interfaces. 2024 Apr 24;16(16):19926-19936. doi: 10.1021/acsami.3c12553. Epub 2023 Nov 17. ACS Appl Mater Interfaces. 2024. PMID: 37975246 Review.
References
-
- Lu X, Sun W, Zheng X, Yang L, Feng T, Deng X, et al. Ruthenium-doped carbon dots with three-in-one chemodynamic, photodynamic, and photothermal activity induce panoptosis for tumor therapy. Chem Eng J. 2025;509: 161355.
-
- Chen W, Feng H, Mo Y, Pan Z, Ji S, Liang H, et al. Hyaluronic acid-functionalized ruthenium photothermal nanoenzyme for enhancing osteosarcoma chemotherapy: cascade targeting and bidirectional modulation of drug resistance. Carbohydr Polym. 2025;349: 122945. - PubMed
-
- Wang MF, Yang R, Tang SJ, Deng YA, Li GK, Zhang D et al. In vivo realization of dual photodynamic and photothermal therapy for melanoma by mitochondria targeting dinuclear ruthenium complexes under civil infrared Low-power laser. Angewandte Chemie-International Ed. 2022;61(38):e202208721. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

