Single-cell transcriptomic landscape of sciatic nerve after transection injury
- PMID: 40849669
- PMCID: PMC12374453
- DOI: 10.1186/s12974-025-03514-3
Single-cell transcriptomic landscape of sciatic nerve after transection injury
Abstract
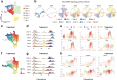
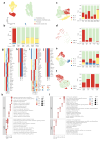
Peripheral nerve injuries, particularly those affecting the sciatic nerve, often result in incomplete functional recovery due to the limited regenerative capacity of adult peripheral nerves. To elucidate the cellular and molecular mechanisms underlying nerve regeneration, we performed single-cell RNA sequencing (scRNA-seq) on rat sciatic nerve tissues at seven time points (Days 0, 1, 3, 5, 7, 10, and 14) following transection injury. Through unsupervised clustering, we identified four major cellular compartments-neurofibroblasts (NFs), glial cells (Glis), immune cells, and vascular cells-and delineated their dynamic trajectories during regeneration. Early responses were dominated by macrophage (Mac) and granulocyte infiltration (Day 1), followed by proliferative expansion of proliferating mesenchymal fibroblasts (NF5) and repair Schwann cells (Gli0) by Days 3-5. Vascular remodeling commenced from Day 7, while Glis progressively transitioned to mature myelinating states (Gli2/Gli5) by Day 14. Pseudotime analysis revealed subtype-specific reprogramming in both Macs and Glis, and cell-cell communication analysis uncovered key ligand-receptor interactions-particularly collagen and PTN signaling between Macs, NFs, and Glis. Bulk transcriptomic validation confirmed sustained and spatially distinct activation of the TGF-[Formula: see text] signaling pathway across cell types and anatomical locations. Comparative analysis with a sciatic nerve crush injury model revealed a stronger early immune response and delayed Gli recovery in transection injury, indicating a narrowed therapeutic window. Together, this work provides a time-resolved single-cell atlas of peripheral nerve regeneration, defines key regulatory circuits within the immune-NF-Gli axis, and identifies phase-specific therapeutic targets-such as early Mac heterogeneity, NF4-mediated matrix remodeling, and Schwann cell remyelination-for enhancing functional recovery following severe nerve injury.
Keywords: TGF−β signaling; Glial cell reprogramming; Macrophage heterogeneity; Nerve regeneration; Neurofibroblast–glia interaction; Sciatic nerve transection; Single−cell RNA−seq.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All experimental procedures involving animals were approved by the International Council for Laboratory Animal Science and conducted in accordance with guidelines for the care and use of laboratory animals. Efforts were made to minimize animal suffering and reduce the number of animals used in the study. All procedures were approved by the Institutional Animal Care and Use Committee of PLA General Hospital (approval number: 2023‑x4‑19) and conformed to national guidelines for animal care. Competing interests: The authors declare no competing interests.
Figures







References
-
- Litovchenko TA, Borodai OM, Tondiy OL. Quality of life of patients with post-traumatic neuropathies and plexopathies accompanied by chronic neuropathic pain syndrome. Int Neurol J. 2021;17:21–4.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

